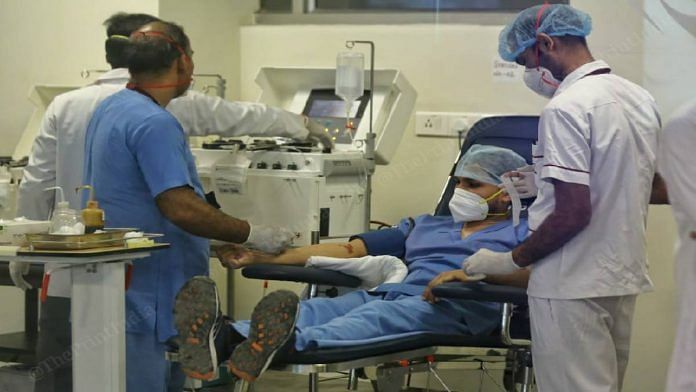
New Delhi: A week after the Indian Council of Medical Research (ICMR) chief Balram Bhargava told the press that convalescent plasma therapy could be removed from the Clinical Management Protocols for Covid-19, demand for plasma — and its administration to Covid patients — continues full steam.
At present, plasma therapy is an investigational therapy permitted “off label”, meaning for unapproved use outside trial settings.
In his comments, Bhargava had cited an ICMR study, published in the British Medical Journal, which found that plasma had no effect on mortality or progression of the disease.
The use of plasma therapy has gone largely unregulated since it was first considered a viable treatment for Covid, with governments and civil society groups making appeals to donors to “save lives” despite any hard evidence.
Doctors across the country, however, continue to prescribe it saying it produces genuine results.
“Until we get a notice from the government, or it is actually removed from the guidelines, we will continue to give patients plasma. We find that it is working very well, as long as it’s given at the right time,” said Dr Rafiq uz Zaman, head of blood transfusions at Apollo Gleneagles, Kolkata.
Here’s a look at how plasma therapy gained popularity, the gaps in its policy, and why doctors still want to use it to treat Covid patients.
Also read: Behind Covid curve or tropical boon? India sees declining cases as US & Europe see surge
‘Holds promise’
Simply put, convalescent plasma therapy involves the transfusion of antibody-rich plasma from a recovered patient into someone who hasn’t been able to mount a sufficient immune response against Covid.
The ICMR’s study, the world’s biggest randomised control trial on plasma therapy, found that two doses of convalescent plasma over 28 days didn’t affect mortality or progression of the disease when given to moderately ill patients. It did, however, ease shortness of breath and other symptoms in a seven-day period. The trial was conducted on 464 participants from 39 hospitals.
The Indian agency isn’t alone in its findings. Observational data from over one lakh patients in the US found a statistically insignificant percentage-point difference in 30-day mortality rate between patients given high and low concentrations of antibodies.
However, the ICMR trial has faced criticism over testing of neutralising antibodies in the donor plasma post-facto. In other words, the level of antibodies in the plasma was checked after it had been donated, reportedly due to lack of testing kits at the time.
Dr Randeep Guleria of the All India Institute of Medical Science (AIIMS) reportedly told the press that it was too soon to write off the therapy, considering “a large number of patients who were given plasma already had antibodies. If you already have, giving it from outside may not be of much use”.
Dr Vikas Maurya, director of pulmonology at Fortis Hospital in Delhi, explained: “There are different levels of recommendations of studies. Studies that have several randomised trials (RCT) are called Level 1, and Level 2 studies are those which have only one RCT… Plasma therapy is a Level 2 study which still needs a metaanalysis and more similar trials.”
During the trial, the plasma was also administered at the moderate stage of the disease — an aspect of the therapy that is still under debate by many doctors. While the ICMR study says plasma therapy does not affect mortality at the moderate stage, there is no clear consensus about whether it would work if given at other stages of the disease.
“The earlier it is given, for example between days 3-4, the results will be positive. In our observations, it is most effective when the case is turning from mild to moderate. If you give it after that, it will be too late and won’t help,” said Zaman.
Dr Ruchi Punamia of KEM Hospital in Mumbai said most doctors aren’t wrong when they say plasma therapy has a positive impact on Covid patients.
“There is no question of it not working. The basics, in theory, are very clear. Plasma includes antibodies which helps the body fight the infection. This is the basic principle on which vaccines are designed, hence it holds promise,” she said.
Dr Vishal Rao, who heads the plasma bank at HCG Hospital in Bengaluru, said plasma helped those who were on the ventilator recover.
“We have found a 60 per cent improvement in patients who are on a ventilator. It’s been helpful for those who are severely ill. Lack of evidence is not the absence of evidence,” he said, adding that it is the only Covid therapy with “medical precedence”.
Also read: ICMR data shows BCG vaccine raises immune response in elderly, could protect against Covid
Unrelenting demand
Kanupriya Mal, founder of Dhoondh, a platform connecting plasma donors with patients, said the demand for plasma is still very much there. “The numbers have dipped slightly since the last five or six days, but that could be due to many reasons,” said Mal.
Dhoondh registers an average of 100 requests for plasma a day from across the country from patients who have doctor’s prescriptions.
The rise of plasma as a viable option has been propelled, in part, by various state governments batting for the treatment without any hard evidence. Even before the Drugs Controller General of India — the country’s apex drug regulation body — approved plasma for “off label” use in June, Delhi Chief Minister Arvind Kejriwal claimed early studies showed “encouraging results”.
“Plasma therapy was promoted by governments so much when none of these statements were evidence based, and no regulatory authority stepped in to monitor this,” an ICMR official in the Covid National Task Force told ThePrint on condition of anonymity.
By June end, the Maharashtra government launched the plasma therapy PLATINA “trial cum project”, which it claimed would “save around 500 lives” through the initiative. More recently, the Andhra Pradesh government promised donors cash rewards while Assam promised preferential treatment in government jobs.
ThePrint emailed a questionnaire about the regulation of plasma therapy to the DCGI, but there was no response until the time of publishing this report.
Also read: 80% Covid patients in a Spanish hospital were Vitamin D deficient, study finds
Continuing gaps in policy
After the guidelines on plasma therapy were released, there was no way to standardise its use. For one, the type of test to check the level, or titre, of antibodies in donor plasma is expensive and difficult to find.
“Only a Biosafety Level 3 lab can do the gold standard neutralising antibody titre (Nab) test. Most labs offering plasma therapy are unlikely to be doing it,” said the ICMR official quoted above.
Second, while the guidelines state the minimum level of antibodies should be 1:640, hospitals are using different standards in absence of the Nab test.
In Apollo Indraprastha, Delhi, for example, the titre used is 1:320. In Apollo Kolkata and HCG Hospital in Bengaluru, the measurement is a US Food and Drug Administration approved test that checks antibody strength by using a cutoff number. In the US, this number is pegged at 12 for donor plasma, indicating it is antibody-rich. In India, there is no standard figure yet.
“Any value greater than 5 is considered effective, so that’s what we are going by. We are getting very positive results,” Zaman said. Rao, who heads the plasma bank at HCG, said they used a value of 11 or higher.
Maurya is of another opinion. “A value of 6 and above is considered a moderate — an amount enough to be eligible for donation. For those in need of plasma, patients with below 1 are given antibody-rich plasma,” he said.
Often, patients are asked to bring their own plasma donors or replacements for the plasma given. Hospitals charge anywhere between Rs 7,500 and Rs 14,000 per transfusion, citing expensive equipment and testing kits. The government of Maharashtra recently put a cap on the price of plasma at Rs 5,500 so hospitals would not make a profit off the treatment.
In some cases, doctors prescribe plasma therapy as the “last resort” even if there is a chance it will not work. “Every helpless doctor and family wants to say they have tried to save a life,” Rao said.
Also read: Modi govt panel seeks ‘justification’ from US firm to test blood thinner in mild Covid cases