New Delhi: The Narendra Modi government’s panel of experts has rejected a proposal by the Mumbai-based Macleods Pharmaceuticals to test the influenza drug, umifenovir hydrochloride, as a possible Covid-19 cure.
The company had in September filed a proposal to check the efficacy of the “single drug”, umifenovir hydrochloride, but sought permission to test it along with favipiravir — the antiviral medication being closely monitored as a Covid-19 ‘cure’.
But the subject expert committee (SEC), which advises the Drug Controller General of India (DCGI), has asked the company to revise the proposal to check for the efficacy of umifenovir alone.
Umifenovir is primarily used for the treatment of influenza, mainly in Russia and China. However, the drug has recently come into prominence worldwide for its potential use against Covid-19.
It is among the other potential antiviral drugs such as interferon, ribavirin, hydroxychloroquine, lopinavir and ritonavir that have been tested on Covid‐19 patients. These drugs were approved based on previous treatment experience of severe acute respiratory syndrome (SARS) and the Middle East Respiratory Syndrome (MERS).
“The firm presented its proposal for clinical trial of Umifenovir and Favipiravir along with standard supportive care versus Favipiravir along with standard supportive care in hospitalised subjects with moderate Covid-19 infection,” the panel said, according to minutes of a meeting on 28 September.
The minutes were uploaded on the website of the Central Drugs Standard Control Organisation (CDSCO) — the health ministry arm that regulates the quality of drugs and vaccines in the country.
After “detailed deliberation”, the committee recommended that the “firm should submit appropriate protocol for evaluation of safety and efficacy of Umifenovir Hydrochloride Capsules as their application is for approval of Umifenovir Hydrochloride single drug”.
The favipiravir-umifenovir combination is already undergoing trials in the country. Glenmark Pharmaceuticals had in May announced to begin the phase III clinical trial to study the combination. The government-run Central Drug Research Institute (CDRI) in Lucknow is carrying out the Phase III trials.
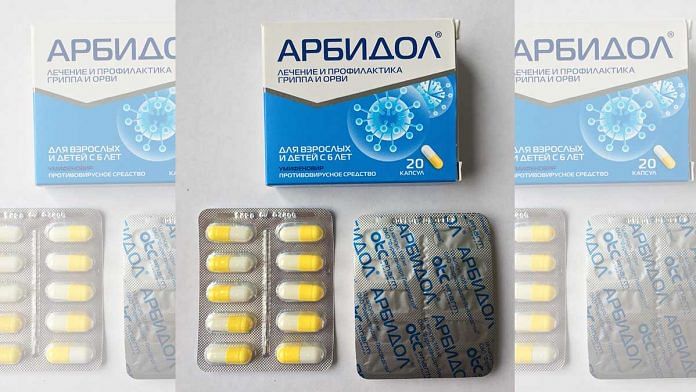
What is umifenovir and why it is being tested against Covid-19
Umifenovir, according to the Journal of Medical Virology, is a broad‐spectrum antiviral agent that could “effectively inhibit the fusion of a virus with host cells”.
Sold under brand name Arbidol, the drug is a “Russian-made small indole-derivative molecule” that was licenced in Russia and China for the prevention and treatment of influenza and other respiratory viral infections.
“Previous research has elucidated that umifenovir is an efficient inhibitor of SARS‐CoV‐2 in vitro (in test tube based studies),” the research published in the journal said.
It, however, also pointed out that “little is known about the actual clinical efficacy of Umifenovir in vivo (in living organisms) due to lack of large‐scale random clinical trials”.
The study titled ‘Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID‐19): A systematic review and meta‐analysis’ published in July has flagged that “some recent small‐scale clinical studies with limited sample size have drawn controversial conclusions about efficacy of Umifenovir for Covid‐19”.
It further shared an instance where “Umifenovir treatment showed a tendency to improve the discharging rate and decrease in mortality rate”. In another instance, however, it said the study demonstrated that “Umifenovir treatment is not associated with improved outcomes”.
Also read: Govt panel rejects Glenmark proposal to test Covid drug favipiravir with steroid dexamethasone