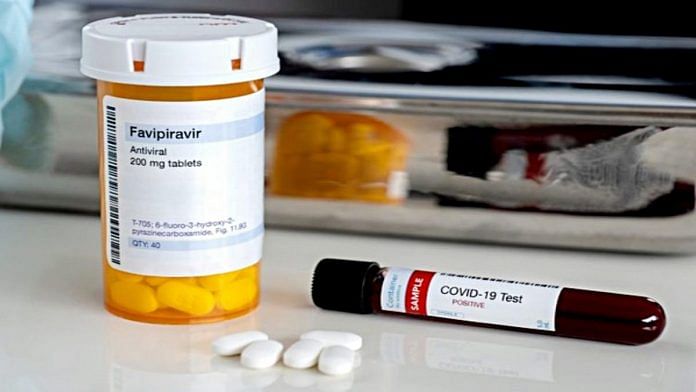
New Delhi: The Narendra Modi government’s panel of experts rejected Mumbai-based pharmaceutical company Glenmark’s proposal to conduct phase 3 clinical trials of favipiravir drug combined with steroid dexamethasone as possible treatment for Covid-19.
Favipiravir, an antiviral medication developed in Japan, is currently the subject of several trials around the world as a potential treatment for Covid-19.
Dexamethasone, a steroid used to suppress skin allergies and treat rheumatic disorders, is part of India’s approved clinical management protocol for Covid-19. It is considered a life-saving drug in Covid therapy after its efficacy in reducing the mortality rate of the disease was proved by researchers at the University of Oxford, who studied the drug as part of the RECOVERY trial.
However, the subject expert committee (SEC) — which advises apex regulator Drug Controller General of India (DCGI) on applications seeking approvals for new drugs, vaccines, and clinical trials for Covid — rejected the drug maker’s proposal to test the combination in phase 3 trials, noting that “the justification presented for use of favipiravir and dexamethasone in all patients of moderate COVID in the test arm is not adequate”.
In phase 3 clinical trials, a drug is tested on thousands to establish safety and efficacy.
The decision was taken by the SEC in a meeting on 23 September. The minutes of the meeting were uploaded on the Central Drugs Standard Control Organisation’s (CDSCO) website — the health ministry arm that regulates the quality of drugs and vaccines in the country.
Also read: Sufficient Vitamin D levels can prevent severity of Covid infection — Boston University study
Concerns raised by high-level panel
At the meeting, the SEC noted that the “dose and duration of Dexamethasone in COVID patients vary with the severity of the disease and other conditions”. However, it said, “in the protocol (submitted by the firm), fixed dose and duration have been proposed for dexamethasone administration”.
The panel also pointed out that the company, in its proposed protocol, has said “the standard arm will not be given steroid which is not as per the National guidelines for clinical management of COVID patients”.
This means Glenmark proposed that the standard arm of the trial — the patients who will be given standard Covid treatment — will not be given steroids. However, the committee noted that since steroids were a mandatory part of India’s Covid treatment protocol, this could not be allowed.
The results of the standard arm are compared to the treatment arm, where participants receive the drug being tested in addition to standard care.
Furthermore, the panel was not convinced with the company’s proposal that “all moderate patients may not require oxygen”, saying that it was not “justified”.
The SEC also did not understand why the firm was testing just dexamethasone when there were multiple steroids that are used in the treatment of Covid patients.
“Other steroids such as methylprednisolone are also recommended for COVID-19 treatment. Hence, use of Favipiravir with Dexamethasone in the proposed study is not convincing.”
The experts said the “firm has already been granted permission to conduct active post marketing surveillance (PMS) study with Favipiravir”. They said the report is awaited.
PMS is also known as Phase IV clinical trials that are done to determine the efficacy of the drug once it is launched in the market for use.
Also read: Depression, OCD, loss of sleep — Covid has made everything worse, but there are ways to cope