New Delhi: The Indian Council of Medical Research (ICMR) clarified Saturday that its call to release an indigenous Covid vaccine, yet to start human trials, by 15 August is only aimed at eliminating red-tape and not bypassing necessary protocols.
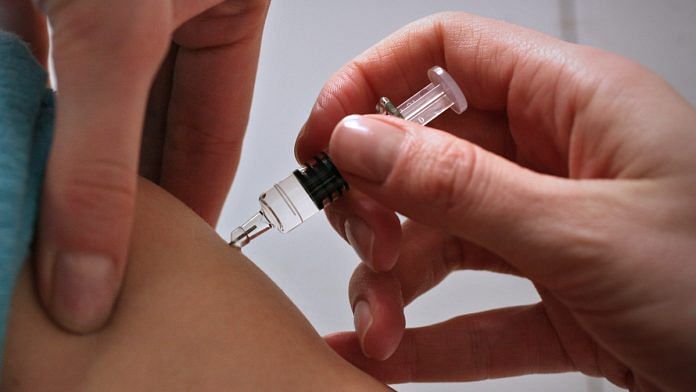
India’s apex medical research agency found itself in hot water as it was reported Friday that it was pushing for a 15 August release for Covaxin, a potential Covid-19 vaccine developed by Hyderabad-based Bharat Biotech International Limited, even though it was yet to start human trials.
The move to fast-track the vaccine manufacturing process had triggered questions about its safety and efficacy, as a submission made by Bharat Biotech to the Clinical Trial Registry of India (CTRI) projected a trial duration of 15 months.
In a press statement issued Saturday, the ICMR sought to address these concerns, saying the vaccine-making process is exactly in accordance with the “globally accepted norms” to fast-track the vaccine development.
It then referred to the letter at the heart of the controversy. In a 2 July missive to 12 institutions selected for conducting the clinical trials, ICMR director-general Dr Balram Bhargava had called for the vaccine to be launched for public health use latest by 15 August 2020.
“The letter by DG-ICMR to investigators of the clinical trial sites was meant to cut unnecessary red tape, without bypassing any necessary process, and speed up recruitment of participants,” the statement said.
“Just as red tape was not allowed to become a hindrance in the fast track approval of new indigenous testing kits or for introducing in the Indian market potential Covid-19-related drugs, the indigenous vaccine development process has also been sought to be insulated from slow file movement,” it added.
Now that preclinical studies have been completed successfully, the phases 1 and 2 (human trials) are to be initiated for Covaxin, it said.
“The aim is to complete these phases at the earliest, so that population-based trials for efficacy could be initiated without delay.”
However, the statement did not clarify whether the institution is still aiming to launch the vaccine for “public health use by 15 August”, which is just over a month away, or if it aims to finish phase 2 trials by the given date.
It said this, instead, “While issues raised in public domain from time-to-time by commentators are welcome, as they form an important part of the feedback loop, the best of India’s medical professionals and research scientists should not be second-guessed for their professionalism or adherence to the highest scientific rigour.”
Also Read: Covid vaccine in India by 15 Aug? ICMR speeds trials as Modi govt eyes Independence Day target
‘Move in the larger public interest’
The ICMR is working with Bharat Biotech International Limited on the vaccine, derived from an inactivated Covid-19 strain.
According to the ICMR, “a large number of vaccines are under various stages of development all across the world”.
“It is also important to promote indigenous vaccine development, while at the same time ensuring safety, quality, ethics and adherence to all regulatory requirements,” it added.
After intense characterisation and review of all data from BBIL, it said, the ICMR is supporting its vaccine candidate’s clinical development as it appears to be promising
The ICMR said their move is “based on in-depth scrutiny of the available data from pre-clinical studies”, which serve as the basis for regulator Drugs Controller General of India’s nod for permissions to conduct phase 1 and 2 clinical trials.
“In the larger public health interest, it is important for ICMR to expedite the clinical trials with a promising indigenous vaccine,” it said.
It said the “ICMR’s process is exactly in accordance with the globally accepted norms” to fast-track vaccine development for diseases of pandemic potential, where human and animal trials can continue in parallel.
“…Faced with the unprecedented nature of the Covid-19 pandemic, and the consequent dislocation of normal life, all other vaccine candidates across the globe have been similarly fast-tracked,” it said in the statement.
Trials, it added, will be done using best practices. “Our trials will be done following the best practices and rigour, and will be reviewed, as required, by a Data Safety Monitoring Board (DSMB),” the ICMR said, describing itself as “among the world’s most reputed organisations in the field of medical research and regulation”.
Also Read: From Phase 0 to long-term study, a look at how long it actually takes to develop a vaccine
Aiming to launch vaccine by 15 August
The controversy around the vaccine started as Bhargava’s 2 July letter, which said the Narendra Modi government is aiming to launch the vaccine for public health use by 15 August, came to light.
“It is envisaged to launch the vaccine for public health use latest by 15 August 2020 after completion of all clinical trials,” reads the letter sent to 12 institutions selected for conducting the clinical trials.
“ln view of the public health emergency due to Covid-19 pandemic and urgency to launch the vaccine, you are strictly advised to fast-track all approvals related to initiation of the clinical trial and ensure that the subject enrollment is initiated no later than 7th July 2020,” the letter states.
It also warned that guidelines and protocols should not be bypassed. “Kindly note that non-compliance will be viewed very seriously. Therefore, you are advised to treat this project on highest priority and meet the given timelines without any lapse,” the letter stated.
Also Read: ICMR wants Covid vaccine by 15 August but documents show it’s a 15-month trial



Desperate situations need innovative and unorthodox solutions. There are several candidate vaccines under development. Normally it would take years to complete the formalities of clinical trials before release. It appears India with the vast population will have the maximum deaths overtaking the US. Add to this the economic misery the country has to go through in the coming years. I would suggest we identify three or four phase 2 cleared vaccins and start vaccinating the vulnerable population on a voluntary basis WITH INFORMED CONSENT. . The initial few thousands of people acting as phase 3 trials study group with those not opting for the vaccine as controls. With weekly reviews and course corrections on the way, we will identify the right vaccine in weeks or months. Government should indemnify the companies against litigation. There won’t be any dearth of volunteers.
Can your journalists write clearly and chronologically without so many confusing repetitions and mystery.
First a brief summary and then details in a proper sequence without repetitions and total confusion. It reflects the situation inside the writers head and the editors inability to deal with it.
God bless the readers.