New Delhi: US health watchdog Food and Drug Administration (FDA) last week banned the sale of pelvic surgical mesh meant for women. Triggered by the move, the Indian regulator is also considering a ban on sales based on the availability of device and import history.
However, the device is not a newly-discovered culprit. It has been under the scanner since 2008 when the USFDA started noticing a spurt in complications related to the device.
Even a Netflix documentary released in 2018, The Bleeding Edge, highlighted the injuries caused by the implant. It also featured prominently in ‘Implant Files’, a global investigation series by the International Consortium of Investigative Journalists, published in November last year.
In the US, seven mesh manufacturers, including Boston Scientific and Johnson & Johnson, are paying almost $8 billion (Rs 56,000 crore) to resolve the claims of more than 100,000 women, reported The New York Times.
With the Indian authorities reviewing their stand on the device, ThePrint explains pelvic mesh implants and their health hazards.
Also read: Modi govt writes to J&J for details on surgical pelvic mesh after US ban
What is transvaginal or pelvic mesh?
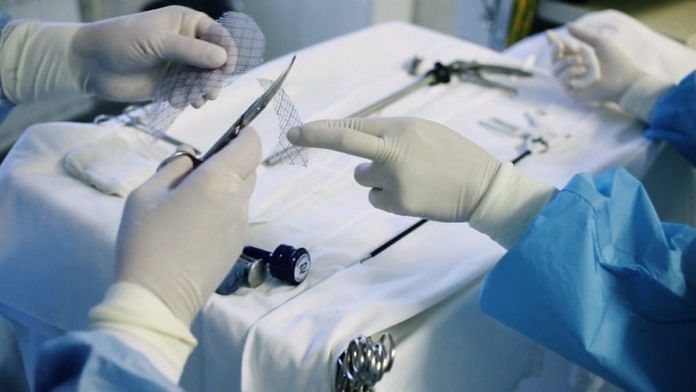
The mesh is a medical device popularly used to support the abnormal descent of the pelvis in women. It is a cloth-like piece of netting made of man-made synthetic material or animal tissue that is used to provide permanent reinforcement to the urogynecologic repair.
What does pelvic mesh treat?
Also known as urogynaecological meshes, the device is used to treat urinary incontinence and pelvic organ prolapse (POP) in women. Both the conditions can develop due to reasons like childbirth, hormonal changes during menopause, heavy lifting or hereditary.
Urinary incontinence is a condition which leads to women leaking from their bladder while doing activities such as sneezing or coughing. According to European Society of Human Genetics, urinary incontinence in women is common, with almost 50 per cent of adult women experiencing leakage at least occasionally.
The meshes are also used for the treatment of pelvic organ prolapse, a condition in which the muscles and tissues supporting the organs at the pelvic floor — the uterus, bladder, or rectum — become weak or get damaged and move out of place.
In both conditions, the surgical mesh can be implanted through the vagina to permanently strengthen the weakened or injured muscles.
According to USFDA, the surgical mesh has been used by surgeons since the 1950s to repair abdominal hernias.
“In the 1970s, gynaecologists began implanting surgical mesh for abdominal repair of POP and, in the 1990s, for the transvaginal repair of POP. In 2002, the first mesh device for transvaginal repair of POP was cleared for use as a class II moderate-risk device,” said the USFDA press statement last week.
When was pelvic mesh first categorised as harmful?
In 2016, the USFDA reclassified the mesh for transvaginal repair of POP into the highest risk class of devices, which requires pre-market approval (PMA) applications — the agency’s most stringent protocol to review the safety of the device.
Almost three years later, on 16 April, it banned the sale of the product in the country.
Before the US, other countries had also taken action to restrict vaginal mesh. In November 2017, Australia banned the mesh deeming it “too risky”. In the United Kingdom and Ireland too, the use of pelvic mesh was suspended in July 2018.
Also read: Sales of pelvic surgical mesh for women could be stopped in India after US ban
What are its complications?
Tens of thousands of women across the world have reported serious complications, including intense pain and bleeding due to the mesh, since the USFDA approved it for vaginal usage in 1992.
Over the years, the mesh has been reported for cutting into sensitive organs of women’s bodies and causing mental trauma due to the loss of job, broken marriages and inability to take care of children.
For instance, the surgeon who first examined UK-based Kate Langley who had undergone the implant previously “could see the [mesh] tape had come through her vagina”.
“The mesh had cut its way through – like a cheese-wire,” Langley said in April 2017.
Another woman and victim of vaginal mesh, Jody Callahan, who had undergone numerous surgeries to remove fragments of mesh from her body, told the USFDA panel of experts at a public hearing in February, “It (mesh) destroys a woman from the inside out.”
In 2017, a survey by Australia-based consumer advocacy group Health Issues Centre said 59 per cent of the respondents out of a sample of 2,200 women said that the procedure did not resolve their original issue.
Status unknown in India
In the US, about 1 in 8 women has surgery to repair POP over her lifetime, and a subset of these surgeries are completed transvaginally with the use of surgical mesh, the USFDA said in its statement.
While the percentage of women undergoing transvaginal POP mesh procedures has decreased in the US in recent years — after the USFDA issued several warning letters about the risks — the number of women treated with mesh in India remains unknown.
It was only last year that the Indian government began to streamline a mechanism of materio-vigilance under which the medical devices will be screened on a regular basis. The trigger was last year’s controversy over Johnson & Johnson’s faulty ASR hip implants.
So there was no stringent mechanism in place to report side effects of the medical devices which Indian women could have used to register their complaints.
Moreover, mesh makers like J&J have conducted a recall from time to time in several countries, but not many recalls have taken place in India.
“We know that several pelvic mesh products that were recalled abroad and implicated in severe, debilitating adverse health outcomes, on large-scale, were also approved in India for several years such as Monarc, Miniarc, Apogee, Perigee by American Medical Systems; Gynecare Gynemesh PS by Johnson & Johnson,” said Malini Aisola, co-convenor of NGO All India Drug Action Network.
“However, no official recall appears to have taken place in India which would have involved providing information to healthcare providers and the public about the hazards of the device and tracing of patients,” said Aisola.



Can I get a recommendation of best mesh removal doctor in India?