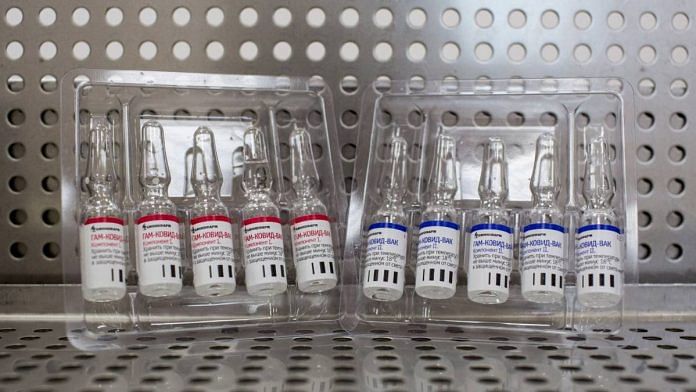
New Delhi: Russia’s sovereign wealth fund, the Russian Direct Investment Fund (RDIF) has reached out to three companies in India — Zydus Cadilla, Wockhardt and Reliance Lifesciences — for manufacturing and trials of Sputnik V, the first registered Covid vaccine in the world.
The Indian government has committed to facilitate the process once any or all of the companies get into a formal arrangement either with RDIF or the Gamaleya Institute that has developed the vaccine, said top government officials.
The RDIF supported the development of the vaccine by the Gamaleya Center and is investing in its mass production. A lot of information, including data from the phase I and II trials, animal toxicity and preclinical data, has already been shared with India and is currently being examined by scientists here. India has also asked for additional data.
“The Russian government has reached out to us for manufacturing of the vaccine and for the conduct of Phase III trials or bridging studies in India. However, for now, RDIF has reached out to three major players in India — Zydus Cadilla, Wockhardt and Reliance Lifesciences — for that,” a senior government official told ThePrint.
“They had asked us about partners and we had told them that it is not the mandate of the government to choose partners for vaccine manufacture. But once any company has entered into an agreement with either Gamaleya or RDIF, we will facilitate the process of clearances,” the official further said, adding that the onus was on companies to move relevant trial applications in India before the clearance process begins.
Also read: Russia in talks with India for ‘Sputnik V tech transfer’ to boost production, exports
India not ‘unduly’ alarmed by concerns on Sputnik V
Russia’s Sputnik V is an adenovirus vector-based vaccine that was registered by the country’s health ministry on 11 August. It is the world’s first registered Covid-19 vaccine, which inspired several misgivings about it before the trial data was published last week.
In the weekly Covid briefing held by the health ministry, NITI Aayog member Dr Vinod Paul said Tuesday, “The government of Russia reached out to the Government of India through appropriate channels and sought help on two counts — one was to consider its manufacturing through a network of companies that are well known for vaccine manufacturing … It is an offer from a friend and one who has been a very special friend of the country.
“So on both these tracks there has been significant movement, there has been outreach extended to several companies in India and two, three, four of them have already come forward and are talking to their Russian counterparts,” Dr Paul added.
Meanwhile, in an open letter, 19 scientists from across Europe and the US questioned the Russian vaccine data published in The Lancet, using phrases like “highly probable”, “strange patterns”, “very strange” and “photoshopped” for the data tables published in the prestigious medical journal.
According to senior officials, India has taken note of these concerns, but is not unduly alarmed.
“It is a highly competitive field right now. In the past with hydroxychloroquine, we have seen how these journals work. So we are not unduly concerned. It is not as if we will stop engaging because of these concerns. We already have some of the data that we are examining. We are awaiting the rest,” said the senior official.
Also read: These are the 3 vaccines Modi says India is ready to produce after go-ahead from scientists
India trying to get details on Astra Zeneca pause on vaccine trial
Astra Zeneca Wednesday announced that it was halting the trials of the vaccine developed by Oxford University after a participant reported an unexplained illness. Government officials told ThePrint that the Indian government is now also trying to get details about the apparent adverse reaction.
Serum Institute of India, which is partnering with Astra Zeneca on the vaccine trials, tweeted that they can’t comment on the trials pausing in the UK, but said the Indian trials are continuing and have faced no issues at all.
We (Serum Institute of India) can't comment on reports of AstraZeneca pausing the trials in the UK, other than that they have been paused for review and shall restart soon. The Indian trials are continuing and we have faced no issues at all.#SII #COVID19 #Latestnews pic.twitter.com/HWPUrQydWc
— SerumInstituteIndia (@SerumInstIndia) September 9, 2020
Also read: India readying roadmap to ensure Covid vaccine reaches all in ‘shortest time’, ICMR says



The subject has to be corrected to RDIF rather than RFID which means something else