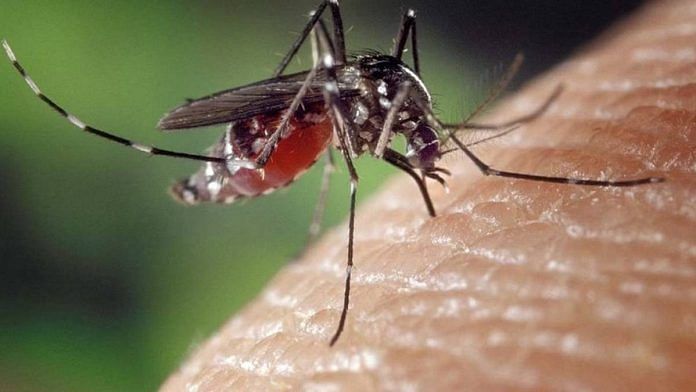
New Delhi: The World Health Organisation (WHO) Monday recommended a new vaccine against malaria, a potentially life-threatening disease caused by parasites that are transmitted through the bite of infected female Anopheles mosquitoes, raising hopes of the disease’s elimination from endemic countries.
The R21 or Matrix-M vaccine, is only the second malaria vaccine in the world to be recommended by WHO and has been developed by scientists at the Oxford University and will be produced by Pune-based vaccine maker Serum Institute of India (SII).
The recommendation for the vaccine in children (the vaccine is for use only in children) comes following advice from the WHO Strategic Advisory Group of Experts on Immunization (SAGE) and the Malaria Policy Advisory Group (MPAG) and was endorsed by the WHO Director-General following its regular biannual meeting held on 25-29 September.
The R21 vaccine is the second malaria vaccine recommended by WHO, following the RTS,S or AS01 (brand name Mosquirix) developed by the British pharmaceutical major GlaxoSmithsKline (GSK), which received a WHO recommendation in 2021.
GSK had tied up with Hyderabad-based Bharat Biotech for the production of the vaccine, but the Indian vaccine maker is expected to supply the vaccine only from 2028. Till then, GSK has reportedly committed to keep supplying the vaccine to several countries through its own production lines.
“Both vaccines are shown to be safe and effective in preventing malaria in children and, when implemented broadly, are expected to have high public health impact,” WHO said in a statement Monday.
GSK, in response to a query by ThePrint, did not reveal the price of the vaccine, but according to information available on the United Nations International Children’s Emergency Fund (Unicef) website, the vaccine will cost EUR 9.30 (Rs 811) per dose for supply during 2023-2025.
In comparison, the price of the new vaccine, according to WHO, has been pegged at US $2-4 (Rs 166-Rs 322) per dose.
SII said in a statement Monday that R21 has recently reached the primary one-year endpoint in a pivotal large-scale phase 3 clinical trial, including 4,800 children, across Burkina Faso, Kenya, Mali and Tanzania.
The trial results are under peer review before publication and the efficacy of the vaccine over 12 months was 75 percent at sites with high seasonal malaria transmission and 68 percent at sites with more perennial transmission using standard age-based administration, it added.
The countries with the maximum burden of malaria include Democratic Republic of Congo, Mozambique, Uganda, Nigeria, Malawi, Angola, Kenya, Tanzania, Ghana and Zambia, among others.
India had reported 160,000 cases of the disease in 2021, but it came down to 45,000 in 2022, according to government estimates.
India has set a target of eliminating malaria by 2030 but so far there is no indication of rolling out a vaccine against the zoonotic disease through the government’s Universal Immunization Programme.
ThePrint explains the significance of the latest WHO recommendation and which regions across the world are expected to benefit from the new vaccine.
Also read: China study links skipping breakfast to increased cancer risk. Need more proof, say Indian doctors
Mosquito-borne ailment that plagues a large part of the world
Malaria is believed to have been existent since prehistoric times and is characterised by fever, fatigue, vomiting and headaches, while in severe cases it can cause jaundice, seizures, coma or death.
The UN health agency’s World malaria report 2022 estimated that globally, there were an estimated 247 million cases of malaria in 2021 across 84 malaria endemic countries, an increase from the 245 million estimate in 2020. Most of the increase was seen in the countries in the WHO African Region.
Twenty-nine countries accounted for 96 percent of malaria cases globally, and four countries — Nigeria (27 percent), the Democratic Republic of the Congo (12 percent), Uganda (5 percent) and Mozambique (4 percent) — accounted for almost half of all cases globally.
According to the report, the WHO South-East Asia Region accounted for about two percent of the burden of the 2021 malaria cases globally and in the region, cases of the disease decreased by 76 percent over a 20-year period, from 23 million in 2000 to a little over 5 million in 2021.
While India accounted for 79 percent of the cases in the region in 2021, within the country, Odisha, Chhattisgarh, Jharkhand, Meghalaya and Madhya Pradesh mostly carry the burden of the disease, with Odisha reporting the highest number of cases every year.
The WHO report also showed that there were 619,000 deaths caused by the disease in 2021 — with over two-thirds of the deaths taking place in children under five years of age. Of the overall deaths, 9,000 were reported from South-East Asia, with India accounting for 83 percent of these deaths.
Current vaccine in use and hope for the future
In October 2021, WHO recommended the RTS,S/AS01 malaria vaccine for the prevention of P. falciparum malaria (the main malaria parasite) in children living in regions with moderate-to-high transmission. In July last year, the vaccine also received its pre-qualification approval.
A WHO prequalification for a medicinal product is a symbol of assured standard of quality, safety and efficacy for procurement agencies.
In response to a query by ThePrint, a GSK spokesperson said in a statement that together with PATH, a global not-for-profit health organisation, the company is donating up to 10 million doses to the ongoing Malaria vaccine Implementation Programme (MVIP).
“We are now increasing our production volumes for roll out beyond the pilot programme, with a plan to supply 15 million doses annually from 2026 through 2028,” it added
The spokesperson added: “We have seen the positive impact of RTS,S through the Malaria Vaccine Implementation Programme in Ghana, Kenya and Malawi, building on the extensive body of data across a range of malaria transmission settings.”
In a statement two years back, WHO said significant reduction (30 percent) in deadly severe malaria was reported following use of the vaccine, even when introduced in areas where insecticide-treated nets were widely used and there is good access to diagnosis and treatment.
“We are delighted that more countries will start implementing vaccination with RTS,S soon, recognising its value in reducing the burden of this devastating disease. GSK will continue working with partners to accelerate additional supply to ensure that even more malaria-endemic countries can roll out RTS,S in due course,” WHO added.
The company, however, did not reveal the cost of the vaccine whose full course includes four doses, saying it transfers technology to Indian vaccine maker Bharat Biotech.
GSK stressed that a phased expansion of Mosquirix is already underway in Ghana, Kenya and Malawi, and, working with partners, it is making good progress towards more malaria-endemic countries being able to roll out the vaccine in due course.
The WHO said Monday, however, that the “demand for malaria vaccines is unprecedented; however, available supply of RTS,S is limited”.
“The addition of R21 to the list of WHO-recommended malaria vaccines is expected to result in sufficient vaccine supply to benefit all children living in areas where malaria is a public health risk,” said the health agency.
Why a new vaccine against malaria could be advantageous
The WHO said that in areas with highly seasonal malaria transmission (where malaria transmission is largely limited to four or five months per year), the R21 vaccine was shown to reduce symptomatic cases of malaria by 75 percent during the 12 months following a 3-dose series.
A fourth dose given a year after the third maintained efficacy till the trial period. This high efficacy is similar to the efficacy demonstrated when RTS,S is given seasonally.
The WHO has also said that at prices of US$ 2–US$ 4 (Rs 166- Rs 322) per dose, the cost-effectiveness of the R21 vaccine would be comparable with other recommended malaria interventions and other childhood vaccines.
The agency, however, also insisted that the two WHO-recommended vaccines, R21 and RTS,S, have not been tested in a head-to-head trial.
“There is no evidence to date showing one vaccine performs better than the other. The choice of product to be used in a country should be based on programmatic characteristics, vaccine supply, and vaccine affordability,” it said.
SII said in a statement Monday that with the approval and recommendations by the WHO, additional regulatory approvals are expected to follow shortly and R21 vaccine doses could be ready to begin wider roll-out as early as next year.
The Serum Institute has already established production capacity for 100 million doses per annum, which will be doubled over the next two years, said the vaccine maker.
(Edited by Poulomi Banerjee)
Also read: ICMR urges scientists to develop accurate typhoid test — ‘existing ones could spur drug resistance’