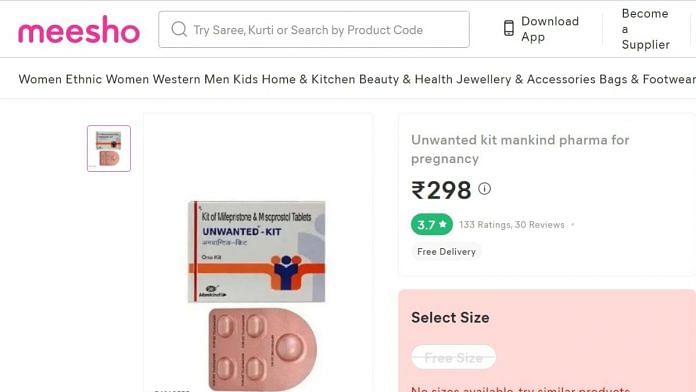
Mumbai: On 29 March this year, Somnath Muley of Maharashtra’s Jalgaon placed an order for Mankind Pharma Ltd’s ‘Unwanted Kit’ (free size), on Meesho, an e-commerce app, through his wife’s account.
The Unwanted Kit, which consists of ‘Mifepristone Tablets IP and Misoprostol Tab IP’ and is used for medical termination of pregnancy, cost him Rs 409 and was delivered on 3 April. And though the kit clearly mentioned that the medicines should not be sold without a doctor’s prescription, Muley was never asked for one while purchasing it.
A day after Muley had placed his order, on 30 March, Monica Dhawad from Nagpur, too, placed an order for the ‘Unwanted Kit’ on Meesho. The kit cost her Rs 310 and was delivered on 3 April.
Neither, though, was looking to use the pills to terminate a pregnancy — Muley, a sub-divisional officer with the Food and Drug Administration (FDA) of Maharashtra, and Dhawad, an FDA drugs inspector, were both posing as customers as part of an operation to bust an alleged abortion pill racket in the state, ThePrint has learnt.
The FDA was acting on complaints it had received from the Chemists and Druggists Association regarding the online sale of abortion kits via the Meesho app without a doctor’s prescription. To check the authenticity of this complaint, FDA officers in 13 parts of the state posed as customers and easily procured the kits.
By 30 April, an FIR was registered against Meesho in Nagpur on Dhawad’s complaint, and another was lodged on Muley’s complaint on 7 May — two of a total 14 that have been registered by FDA officers under the Drugs and Cosmetics Act, 1940, against the sale of pregnancy termination kits online.
“These pills are categorised as ‘scheduled H’ class of prescription drugs and are given only after a doctor’s prescription. Selling them without any prescription is playing with a girl’s or a woman’s life and is illegal,” the FIR lodged in Jalgaon, accessed by ThePrint, stated.
In another incident on 6 May in Ahmednagar district, a delivery of two boxes containing a total of 1,800 ‘Avort kits’, amounting to a total of 9,000 abortion pills, was received at the transport godown of the Maharashtra Industrial Development Corporation (MIDC). The invoice described the contents as a different brand and type of drugs. The boxes have been confiscated by the Ahmednagar MIDC.
An FIR against the company receiving the delivery and the one that had sent the consignments, was registered by the Ahmednagar MIDC police on 10 May. ThePrint has accessed a copy of the FIR.
“The medicine itself was not illegal in any of these instances, but these medicines are given only with a doctor’s prescription,” D.R. Gahane, joint commissioner, FDA Maharashtra, told ThePrint. “Without such a prescription, taking these pills could be dangerous or even fatal. We, along with the police, are investigating whether this is part of a bigger racket and who all are involved in this.”
In an emailed statement to ThePrint, Meesho said: “Meesho operates a pureplay marketplace, this means we provide a technology platform through which sellers can list their products and customers can make a purchase. The company does not own or stock inventory.
“Meesho qualifies as an intermediary under the Information Technology Act and the Consumer Protection (e-commerce Rules), and in full compliance with our legal obligations, we require all registered sellers to accept our supplier agreement, which clearly specifies that suppliers are not permitted to list any illegal products for sale on our platform.”
The statement further said: “In the instant case, certain sellers had listed abortion kits under wrong names, to circumvent quality checks which flag any products listed with the words ‘abortion’, ‘medical termination’, ‘termination of pregnancy’, etc. As soon as this came to our attention, we immediately delisted these products and removed the concerned suppliers from our platform.”
The e-commerce platform added that they are cooperating with the investigation. “We have been cooperating with the Drugs Control Department and the police in their investigations. Meesho is a law-abiding entity and we will always comply with applicable laws and are also constantly improving our quality check systems to tackle such unscrupulous suppliers who are acting against law.”
Also read: Why India’s law on abortion does not use the word ‘abortion’
‘Playing with the lives of girls, women’
According to the FIR registered by the Ahmednagar MIDC police, FDA officer Javed Sheikh had received a call from the MIDC on 5 May alerting him about the consignments regarding which they had received an anonymous call.
Upon checking, it was found out that the boxes containing 9,000 pills, worth Rs 7.6 lakh, had been delivered from Haryana. However, the invoice mentioned the contents as ‘ORCINACP Tab 2’, ‘ORACLAM 625 Tab’.
The consignments, sent by IVA Healthcare in Panchkula, Haryana, were to be delivered to ‘Shriram Agency’, but when Sheikh, accompanied by the Ahmednagar police, went to check the address mentioned, the agency did not exist at the location, the FIR states.
When contacted, the owner of Shriram Agency, Nitin Bothe, said that though the boxes were addressed to the company, he was not aware they contained abortion kits, the FIR reads. However, upon further investigation, “it was found that Shriram Agency’s owner Nitin Bothe was operating from a different address, which was not licensed, and it was found that he was doing business of such illegal sale of abortion pills using an unlicensed place,” said the FIR.
“Across the state, FIRs have been registered against companies from where the kits and pills are delivered, as well as the Meesho app. Further investigation is on,” said FDA joint commissioner Gahane.
An FIR has been registered against Bothe and IVA Healthcare under sections 18 (c )18 (a) and 22 (cca) of the Drugs and Cosmetics Act.
“Bothe had applied for anticipatory bail, which we cancelled,” said S.P. Patil, DCP Ahmednagar. “We have even sent our team to Haryana and further investigation is on. Bothe is currently absconding. But we will make arrests shortly in this case.”
The pills, the FIR said, are under the “scheduled H prescribed pills category” and are given only after a doctor’s prescription. “Selling them without any prescription is playing with girls’ and women’s lives and is illegal,” it added.
In the Meesho case, the FIRs have been registered under several sections of the Drugs and Cosmetics Act, including 18(a)(vi), 18(c), 27(b) (2), 27(d); section 276 (whoever knowingly sells, or offers or exposes for sale, or issues from a dispensary for medicinal purposes, any drug or medical preparation, as a different drug or medical preparation) of the IPC, and sections 3, 4 and 5 of the Medical Termination of Pregnancy (Amendment) Act, 2021.
(Edited by Zinnia Ray Chaudhuri)
Also read: India and US went pro-choice around the same time. Only one strengthened its abortion laws