New Delhi: In 2021, a group of researchers in India, searching for the origins of a mysterious pathogen that had swept through hospitals across the globe in the last decade, made a breakthrough.
They were looking for strains of a fungus called Candida Auris, which was first discovered in the ear canal of an elderly Japanese lady in a Tokyo hospital in 2009. In the next ten years, the fungus simultaneously appeared on four different continents; each of those four strains was genetically unique enough to convince scientists that this wasn’t a case of the pathogen spreading from one continent to another. Where did the fungus come from? Why was it multi-drug resistant? Why was it so well adapted to causing infections whereas other fungi of the same family, freely spread in the environment, did not infect humans? These were, and are, some of the questions scientists and the medical community have been grappling with, even as Candida Auris has become a cause of chaos and mortality in ICUs across the world.
The researchers combed the length and breadth of the Andaman islands, collecting soil samples from 48 different locations—coastal wetlands, rocky shores, sandy shores untouched by people, beaches frequented by tourists, tidal marshes and mangrove swamps.
What they found offered a vital clue about Candida Auris’s emergence—the scientists isolated two different strains of the fungus. One, from a beach frequented by people, was a multi-drug resistant variety that thrived in hotter temperatures that would kill other Candida species. Another variety, which was neither drug resistant nor heat-adapted, was found in a marshy, non-inhabited area.
“What that suggests,” said Arturo Casadevall, chair of Molecular Biology and Immunology at the Johns Hopkins University in the US, and one of the world’s foremost researchers of fungal diseases, “is that Candida Auris was an environmental fungus of no concern to us, which, due to global warming, had adapted to surviving at higher temperatures and become a pathogen.”
In March, the Centre for Disease Control (CDC) in the US issued a warning about Candida Auris, noting its extraordinary mortality rate—between 30 to 50 per cent of infected people die of the disease it causes—its resistance to drugs, and the startling speed with which it was spreading in ICUs across the US.
Fungal diseases, unlike those caused by viruses or bacteria, have always flown under the radar in medical science. They are non-communicable, and consequently, there is no mandate for hospitals or governments to report the number of cases. Diagnosis is difficult and often requires invasive tissue collection. But the most important reason for its unimportance is that, of the estimated 12 million species of fungi that exist, only a handful infect humans, and most of those cause pesky but non-threatening conditions like dandruff, thrush or athlete’s foot.
We have a natural protection against fungal invasions—the vast majority of fungi do not survive at the internal temperature maintained by mammals. Few that do, are easily overpowered by the mammalian immune system. This may be one reason why we humans exist at all in the first place.
A hypothesis called the “fungal infection-mammalian selection” proposes that the thermal barrier may be responsible for why mammals replaced dinosaurs as the dominant large animals on the planet.
“After the mass extinction event of dinosaurs, why wasn’t there a second reptilian age,” Casadevall, who originally proposed the theory, asked. “And why is that mammals, who are hot, and subsequently need so much food as energy, came to proliferate? We know that there was a massive fungal bloom at the end of the Cretaceous period after the extinction event. We think that’s what selected mammals because mammals could survive that so much better than reptiles.”
He added that Candida Auris may be signalling a change in that equation, as the first example of a fungus that has “adapted to a world being heated by climate change, and breaking through our thermal barrier”.
It’s only late last year that the World Health Organization first recognised fungal diseases as a cause for concern, publishing the first “Fungal Priority Pathogens List”. Candida Auris tops the WHO’s list of pathogenic fungi, along with Aspergillus Fumigatus and Cryptococcus Neoformans. The latter are common moulds found in soil and decaying vegetation which do not usually infect healthy humans, but can cause deadly infections in people with compromised immunity, like those with HIV, organ transplant recipients, or patients being medicated with corticosteroids.
“The estimate is that around 1.5 million people die globally every year from fungal infections,” said Arunaloke Chakrabarti, India’s leading researcher of fungal diseases, who recently retired as head of the department of medical microbiology at PGIMER Chandigarh. “But the actual numbers must be much higher because most people who die of fungal infections already had some other major health problem, so hospitals will list that—transplant failure, for example—as the cause.”
Candida Auris is already a well-established threat in India. In 2015, a study that looked at 27 ICUs at major government hospitals in the country found that it was the number one cause of septicaemia (poisoning of the blood) deaths in ICUs.
Chakrabarti, like Casadevall and many other scientists who work with fungi, believe that we are at a tipping point: that as fungi adapt to climate change, they will cause unprecedented disease outbreaks.
Fungi, like bacteria, are fundamental for life on Earth. It’s fungi that broke down rocks into soil to create the planet as we know it. On a less planetary level, fungi are the reason we have bread, or beer, or bio-diesel. It’s also the only organism known to science that can break down plastic.
“Fungi did not come to destroy us,” Chakrabarti said. “We have done this to ourselves through global warming. There will be havoc, and very soon.”
What would that “havoc”—a deadly fungal outbreak—look like? We don’t need to imagine it, or resort to science fiction like the video game and TV series The Last of Us, because it has already happened in India.
In 2021, just as the lethal Delta wave of Covid-19 was abating, doctors in hospitals across India were startled to discover that an outbreak of mucormycosis, erroneously given the popular moniker “black fungus”, had begun.
India is no stranger to mucormycosis—it is the country with the highest rate of this rare infection (more than 70 per cent of global mucormycosis cases are in India). It has a mortality rate of over 50 per cent.
Even then, doctors view mucormycosis as a truly rare disease, because it infects only the severely immunocompromised, like organ recipients. There has never been an outbreak of the infection in India, till the Delta wave, where even the general population, their immunities hammered by both Covid-19 and the large-scale use of steroids against the disease, became vulnerable to fungal infections. An estimated 50,000 people were hospitalised with mucormycosis, and over 4,500 deaths were recorded.
Through detailed interviews with a group of doctors at a major hospital in New Delhi who were at the frontline of the battle against mucormycosis, a survivor of the infection and her family, and experts like Chakrabarti, who headed India’s mucormycosis task force, here is a look at what it was like to be inside the outbreak.
What is mucormycosis?
Mucormycosis is a rare fungal disease that is caused by the growth of fungus belonging to the Mucorale family inside the human body. It may be rare, but it’s deadly. Mucorale fungi are actually all around us—it’s in the soil, it’s in the air, it helps leaves decay, and we are all inhaling the fungal spores all the time.
“Our immune system clears it easily, so it doesn’t harm us,” said Dr Jatin Ahuja, an infectious diseases specialist who was in charge of the Covid-Associated-Mucormycosis (CAM) protocol for Apollo Hospital in New Delhi. “But untreated diabetes, long use of steroids to suppress immune function, transplant surgeries, chemotherapy…these are things that can lower immunity to a level where mucormycosis can infect a person. Aspergillus is another common fungus that behaves the same way, but mucormycosis spreads much faster.”
Both are deadly if left untreated. If it manages to infect a person, the fungus usually takes hold in the nose and moves through the sinus cavity, eating through anything that comes in the way—tissue, bone, vessels.
“From there it moves into the orbital cavity [eyes],” said Dr Ahuja. “Then it follows the optic nerve into the brain. At this stage, it’s usually too late to save the patient.”
Saving someone infected with mucormycosis requires early clinical diagnosis, but that’s hard because the first symptoms are things most people would ignore—facial pain, blocked nose, headaches, red eyes.
“But the fungus can go through the sinus and take the eye out in the space of five days,” said Dr Ahuja.
Once diagnosed, mucormycosis needs aggressive surgical removal, a line of treatment indistinguishable from that for cancer.
“We remove tissue and bone with a good margin because it’s never completely clear how much the fungus has spread,” said Dr Girish Raheja, senior consultant surgeon with Apollo’s ENT department. “And you pray it does not recur.”
Surgeons like Dr Raheja or his colleague Suresh Naruka, usually get called for one or two mucormycosis cases in a year, but during the outbreak, they were overwhelmed.
“I saw 150 cases in those four months,” Dr Naruka said, “and I operated on over a hundred people. I did six orbital extractions [removal of the eye]. Not all patients survived.”
The initial signs that something unusual was happening came as early as February 2020, when the first wave of Covid hit India. Dr Chakrabarti met me in his office, the only operational room in a cavernous hospital in Rishikesh that was closed during the pandemic, and which he has been tasked with reopening. He was at PGI Chandigarh, one of the largest hospitals in north India during the start of the pandemic, where he ran a centre dedicated to fungal diseases. He noticed a sudden spike in Mucormycosis cases.
“Then we got a report that between three eye centres in Bengaluru, they had reported 18 mucormycosis cases,” he said. “Very, very unusual. I started notifying people in my fungal network around the world.”
Dr Chakrabarti runs a group called Fungal Infection Study Forum, and in September 2020, they started a study across 16 hospitals in India and realised that there was double the number of mucormycosis cases compared to the same period in 2019.
“I’ve never heard of anything like it,” Dr Chakrabarti said.
The research also found that two-thirds of the mucormycosis patients had uncontrolled diabetes, and 80 per cent of the patients had been given increased doses of steroids to treat Covid.
“For the same reasons, aspergillus infections were also on the rise, hospitals were doing sinus surgeries more than ever before,” Dr Chakrabarti said. “As we were finishing the study, the Delta wave was upon us.”
Also Read: No, Covid vaccines didn’t cause those heart attacks. Don’t let hysteria win
The Delta wave hits
Seema Verma, 57, is the matriarch of a bustling joint family living in Faridabad. In April 2021, as the Delta wave of the pandemic swept through India, overwhelming the country’s medical infrastructure like never before, Seema was struggling with a persistent cough. On 23 April, Seema’s son Yash, a software engineer and bodybuilder, decided that she needed to see a doctor. It took them more than two hours, driving around empty streets and highways before they found a clinic that was open. Seema and Yash were in for a shock—her oxygen saturation was at 60 per cent—she was near death (anything below 88 per cent is dangerous and demands immediate hospitalisation).
“I thought she would die,” Yash said.
Yash and Seema were back on the road, driving for hours, looking for a hospital bed and being turned away. They finally found a bed in a small hospital. But the facility did not have oxygen.
“I called friends, family, everyone I could think of to try and get oxygen,” Yash said. “I put requests on Whatsapp groups. I was told to go to a factory in one sector in Faridabad to pick up empty cylinders and then go to another factory which had an oxygen plant to get the cylinders filled.”
Seema and Yash’s experience was not the exception but the norm in April and May of that year. It was also the staging ground for the mucormycosis assault that was to come.
“We were overwhelmed by the Delta wave before we knew what was happening. All the ICUs were full, there was no space anywhere. All the doctors were working all the time,” recalled Dr Sudha Kansal, who was in charge of ICUs for Apollo during the pandemic. “Our phones were ringing all through the day and through the night. We were consulting for 20 hours a day. By April, all the hospitals were running out of oxygen. In the ICUs, we have two oxygen supplies for each bed as a failsafe. We created extra beds between beds and gave patients the extra oxygen supply. I don’t know when I was sleeping. We had no days off. For two months this was what it was like. There were days when I saw 80 patients.”
Seema’s condition showed no signs of improvement; the small hospital she was at had run out of everything—oxygen, medicines, manpower. Yash got a call from a friend who was in a government hospital, telling him that the patient next to him had died, and the bed is now empty. He rushed his mother to the hospital.
“Now I was finally on steroids, and I started improving,” Seema said.
Yash’s thoughts and days were consumed by just one task—getting oxygen cylinders.
“There was one day when I stood in a line for twelve hours before I could get a cylinder,” he said.
On the other side of the fence, doctors were in the fight of their lives too. Not only were they crushed by the sheer number of patients, they were also working without a break, pulling shifts of twelve hours or more day after day.
“The biggest challenge was that I knew I was going to give my family Covid,” Dr Ahuja said. “I got Covid four times, and each time my family got it too. My wife is an internal medicine doctor and we have two children, five and eight. My brother had very severe symptoms and eventually long Covid. My father and I had severe symptoms.”
One day, while he was recovering from Covid himself, Dr Ahuja realised that he had not spoken to his parents in more than five days. He drove to his parents’ house to check on them. He found his brother had an oxygen saturation of 82 and his father registered 85.
“Their CT scores were bad, but I knew there were no beds anywhere, I could not get them admitted,” Dr Ahuja said. “To be frank, I did not want to admit them either because the situation at hospitals was horrible. Complete chaos. Every doctor was looking at 50 patients. There was a feeling of impending doom.”
Seema watched with horror as tragedy unfolded around her in the government hospital, where she was admitted for ten days.
“The person on my left died,” she said. “The next day the person on my right died. Another person came on that bed, and died a day later. I was very scared.”
The deaths during the Delta wave, said Dr Suranjit Chatterjee, who coordinated Covid-19 as well as CAM treatment at Apollo, “really hurt us.”
“Having been a doctor for so many years, losing patients is a daily thing, and maybe we get emotionally blunted to that a little and we can pardon ourselves by knowing that we gave the patient the best treatment we could have,” he said. “But here, we were doing everything, and no matter what we were doing, we were constantly losing. Families would be begging and pleading with us, little children crying, saying, ‘save my parents I have no one else’…obviously it took a toll. There was no peace of mind.”
The doctors, like everyone else during the Delta wave, lost family and friends to Covid. On 6 May, Dr Raheja’s mother passed away from Covid while he was working a shift at the hospital.
Also Read: Covid has surged, but there are hardly any ‘black fungus’ cases this time — doctors explain why
Delta recedes, Mucormycosis moves in
By July, Seema had recovered from her battle with Covid, but the war was far from over for her. She was admitted that month again after she developed breathing difficulties. Scans showed a pneumonia patch in the lungs.
“For fifteen days, I had a pipe through my side into my lungs to drain fluid and then flush it with Betadine,” Seema said. “It was the most painful thing I have ever faced.”
She also had pain and swelling in the face and the gums and had difficulty eating. The doctors suspected TB and extracted spinal fluid for tests. They were not too worried about the gum swelling, even though weeks of medicines had not done anything to alleviate it.
“How do you bear all this?” Yash asked his mother one day. “She told me, ‘I won against Covid, but this is defeating me.’ We did not know that worse was to come.”
As Seema was suffering and fighting, doctors across India were seeing the first signs of people coming in with fungal disease. Blocked noses that would not clear for weeks. Headaches that did not go. Swelling of the face.
“We did scans and realised this must be fungus,” said Dr Raheja. “But I thought, this can’t be right. Why would so many people get fungal infections? What kind of fungus was it?”
At PGI Chandigarh, Dr Chakrabarti was alarmed. There was an unprecedented spike of fungal cases at the hospital and he had identified it as mucormycosis. He called the Indian Council of Medical Research (ICMR) and urged them to send out an advisory.
Soon, reports of mucormycosis were coming in from everywhere in the country.
“I have never seen anything like it,” Dr Chakrabarti said. “A national task force was created and I was the expert on that force. I told them to make it a notifiable disease, and declare it an epidemic, which the government did. It was the first time a fungal disease was notifiable. We needed the numbers. We needed to know what was happening.”
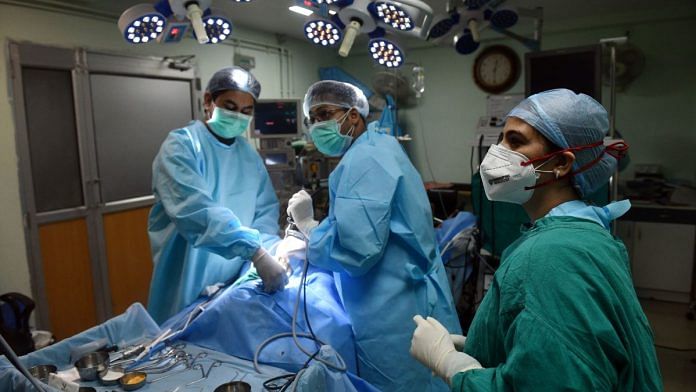
With barely any warning, the doctors at Apollo, which was still working as a Covid-only hospital, went from doing no surgeries to scheduling as many as could be fit into a day.
“Each of us (surgeons) were doing four to five procedures a day,” said Dr Raheja. “Horrible, mutilating surgeries. Taking out eyes, jaws, nose, and half their faces…mucormycosis is literally treated like a cancer. You have to be radical with your surgery otherwise you will lose the patient. You may lose the patient despite your best effort. But I do feel bad, I feel the trauma of the gross disfigurement from the surgeries.”
There are two factors that prevented doctors from acting earlier than they did as the outbreak unfolded. One, the rarity of mucormycosis meant that most doctors simply did not think of it when trying to make a diagnosis. The second is that fungal infections are notoriously difficult to detect.
“Most of these fungi already exist in our nose and airways anyway without becoming infectious,” Dr Kansal said. “So we need to do invasive tests to extract deep tissue and check for fungus there, as well as see tissue damage on CT scans and deduct from that. But once we knew that mucormycosis cases were happening in unusual numbers, we started catching it early in the ICUs. Pain in the nose? Refer to ENT, do an MRI. We could treat it while it was still in the upper airways, not in the lungs, or the brain, when mortality is 95 to 99 per cent.”
The extreme rate of surgeries and their harrowing nature was taking a toll on the surgeons. Dr Naruka recalled one case that made a deep impression on him, a patient who came from UP with CAM. He first did a “debridement” of the sinus cavity—a major surgery under general anaesthesia that involves removing sinus-infected dead tissue—hoping that the fungus had not made its way deeper (scans don’t show fungi, which is microscopic, but only the extent of tissue damage). When he went to check on his patient the day after the surgery, Dr Naruka found that his left eye was red and bulging. A sure sign that the fungus has reached there. He did an emergency surgery to remove the eye. The next day, the fungus was in the right eye. Another emergency operation followed to remove the eye.
“The third surgery, which was done by me and the neurosurgeon, lasted seven hours,” Naruka said. “Now there was no more we could do. Either the fungus was already in the brain, in which case he would die, or we had managed to stop it and he had a chance to recover.”
Three days after the last surgery, Dr Naruka did a final procedure to remove any tissue in the sinus and optical areas that looked unhealthy. The patient survived.
“I sometimes operated on five-six people a day, starting at eight in the morning, finishing after nine at night, two-three hours per surgery, one break to change PPE after six-seven hours, go to the loo, eat something, drink water,” Dr Naruka said. “I would collapse at the end of it. The sisters [nurses] were collapsing in the hallways. I thought, when will this nightmare be over?”
Yash was woken up at 2 am one night by Seema, who was in unbearable pain from the swelling in the mouth. This time, Yash rushed her to Apollo. A CT scan revealed that her jaw bone had been eaten away by fungus. A surgery was scheduled for the very next day.
“When I asked Dr Naruka what her chances were, he said 60-40. Sixty per cent she does not make it,” said Yash. “I told no one in the family about this.”
Seema remembers being wheeled into the OT at 2 pm and then regaining consciousness at 8 pm.
“We had to remove her upper jaw and her palette,” Dr Naruka said. “She was close to losing an eye, but thankfully that did not happen. We did major sinus debridement.”
A successful surgery is just the first step in the treatment of fungal infections like mucormycosis because it requires a long course of anti-fungal medicines after, medicines that cause serious side effects. Fungi, like all plants and animals, and unlike bacteria, are eukaryotes, organisms whose cells contain a nucleus, which means what is poison to fungi is also poison for us.
“On top of that, there are very few antifungals in the world because there is very little research and investment in fungal diseases,” said Dr Chakrabarti. “Amphotericin B is the only drug that works against mucormycosis for example. It costs Rs 10,000 to 15,000 per dose. And naturally, when the mucor wave hit, we faced a severe shortage of Ampho-B.”
Ampho-B also requires careful intravenous administration, said Dr Ahuja, who oversaw the use of the medicine at Apollo.
“We worked seven days a week through the mucormycosis outbreak. Stay till late at night and come back early in the morning. We were already in bad shape from the Delta wave, he said. “Sometimes Ampho-B is rejected by the body. Even if it is accepted, there is a massive electrolyte imbalance, kidneys are badly affected, the superficial veins start clotting easily and it becomes hard to find a vein to administer the medicine.”
For Seema, whose veins dried up quickly once the Ampho-B was started, Dr Ahuja had to find new places to administer the IV every day, including through the veins in her legs.
“I still have pains all over my veins,” Seema said, sitting on a bed in her home, smiling through her story, happy to be alive, with her son sitting next to her. “I lost all my hair. All this hair you see,” she pointed at short, salt-and-pepper hair, “it’s all new! I was so weak I could not stand. I was 70 kg before I had Covid. After I came back from my mucormycosis surgery, I was 49 kg.”
For the next year, Seema had to undergo blood tests every week, and CT scans every month to check for any recurrence of fungus, as well as reconstructive surgeries for her jaw and palate.
Her survival and her spirit was an inspiration to her family—“she is a superwoman,” said Yash—as well as to her treating doctors.
“Every time I went to the hospital, the doctors said, ‘look, it’s Jhansi ki Rani’,” Seema said, laughing.
What caused the outbreak?
The extraordinary, never-seen-before mucormycosis outbreak spawned in its wake an explosion of theories trying to explain what may have caused it. Was it the tainted industrial oxygen people were forced to use when the Delta wave led to an acute shortage of medical-grade oxygen? Did it come from the humidifiers that were suddenly deployed 24/7 at hospitals? Was it in the AC vents in hospitals? Did India’s extreme air pollution have a role to play? Or the religious practice of applying cow dung on the body in a mistaken belief that it prevented Covid?
Dr Chakrabarti and his fungal research group conducted exhaustive studies over the last year into each of these claims.
In the end, the research came to a firm conclusion. There were two main triggers that launched the mucormycosis outbreak — Unchecked diabetes, and over-use and unsupervised use of steroids during the pandemic, said Dr Chakrabarti.
The two reasons faithfully follow two of the biggest faultlines in Indian healthcare. India is second only behind China in leading the diabetes epidemic that has engulfed the world in the past decades, with almost half a billion people suffering from the disease. Second, the indiscriminate availability of drugs (no prescriptions required, or sometimes, prescribed irresponsibly by doctors habituated to doing so) that has led to India becoming the world’s most significant wellspring of multi-drug resistant pathogens.
“Diabetes causes hyperglycemia (high blood sugar). Covid-19 attacks beta-cells in the pancreas which produce insulin, so the body can’t produce enough of it, which also leads to hyperglycemia. Steroids also cause hyperglycemia. And there was stress, which increases the level of a hormone called cortisol in the body, which can also lead to hyperglycemia. When hyperglycemia happens, the iron levels in the blood increases, and iron is great food for mucormycosis,” said Dr Ahuja.
“You know what else is great food for mucor? Zinc,” said Dr Chakrabarti. “And people were self-medicating or being prescribed zinc throughout the pandemic even though there was no proof that it helped.”
Dr Chakrabarti pointed out that the overdosage of steroids, which suppress the body’s immune response and open a pathway for fungus to take hold, happened not only because people took them at home unsupervised and unprescribed in the panic of the pandemic, but also because hospitals, overwhelmed with dying patients, had little option but to push the limit with steroids to try and save lives.
“We were battling helplessly to stem the disaster,” Dr Kansal said, agreeing with Dr Chakrabarti’s assessment. “We knew if we didn’t up the dose of steroids, the patient would die in the next few minutes or hours. There was no point thinking of what may happen later. It’s like we were in a storm, where everyone was just being carried away without knowing where they would reach.”
By August, the mucormycosis wave was over, stopped in its track by hospitals enforcing certain protocols. The first step was the awareness that the disease was indeed mucormycosis, and subsequently, diagnosing it aggressively.
“And then all the hospitals became very, very careful about steroid use,” Dr Chakrabarti said, “and they started monitoring blood sugar constantly. It took us three months to get the outbreak under control.”
It also helped that the deadly Delta wave had receded, leaving doctors with more breathing space and less compulsion to use high steroid doses.
India has all the problems that can lead to fungal outbreaks in the future, said Dr Kansal. “A huge population with uncontrolled diabetes, indiscriminate use of antibiotics, and over-the-counter availability of steroids.”
Globally too, there is cause for not just concern but alarm.
“With global warming, we are expecting to see more and more deadly fungal infections,” said Dr Chakrabarti. “Because fungi will adapt to the heat, and we will lose our thermal barrier against it. Already there is no way to stop Candida Auris from spreading in hospitals. What will come next?”
(Edited by Theres Sudeep)