New Delhi: Top Indian pharmaceutical companies have appealed to the Union government to stay firm on the Patents Act as it engages with the UK, US and the European Union on proposed Free Trade Agreements (FTAs), expressing apprehensions that such deals may adversely impact affordable healthcare, ThePrint has learnt.
India has been in talks with the UK, USA and EU over FTAs for the last several months, and the deal with the UK is expected to be signed this year. Once India signs these pacts, it will have to amend its patents law. The FTA will also override the trade obligations under the World Trade Organisation’s agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS), to which India is a signatory.
The Patents Act, 1970, ensures that patents are not exploited contrary to the national interest and provides shortened patent terms for products. Moreover, India, as a TRIPS-compliant country, grants protection to any pharmaceutical product of 20 years from the patent filing date.
In a representation sent to the Union Ministry of Health and the Department of Pharmaceuticals (DoP) last month, drug companies have stated: “In the recent talks…there is a provision to extend the monopoly of patent holders by extending the life of a patent on a medicine beyond 20 years, thereby delaying entry of generic medicine.”
In the representation, the companies have said it’s common practice among some innovator companies to create minor variations to proprietary medicines, seek another “full” term of patent protection on these trivial modifications, and repeat this process for as long as possible.
According to media reports, there is also talk of introduction of the Regulatory Data Protection system, commonly known as “data exclusivity”, for new drugs in the trade deals with US, UK and EU.
Data exclusivity is a type of intellectual property protection which ensures that a generic drug manufacturer cannot rely on an innovator firm’s data for a specific period for gaining drug approvals, thereby slowing the entry of generic drugs in the market.
“This is beyond TRIPS agreement which stipulates the protection of test and other data for new chemical entities required to be submitted for regulatory approval from unfair commercial use,” the Indian companies have said in their representation to the government.
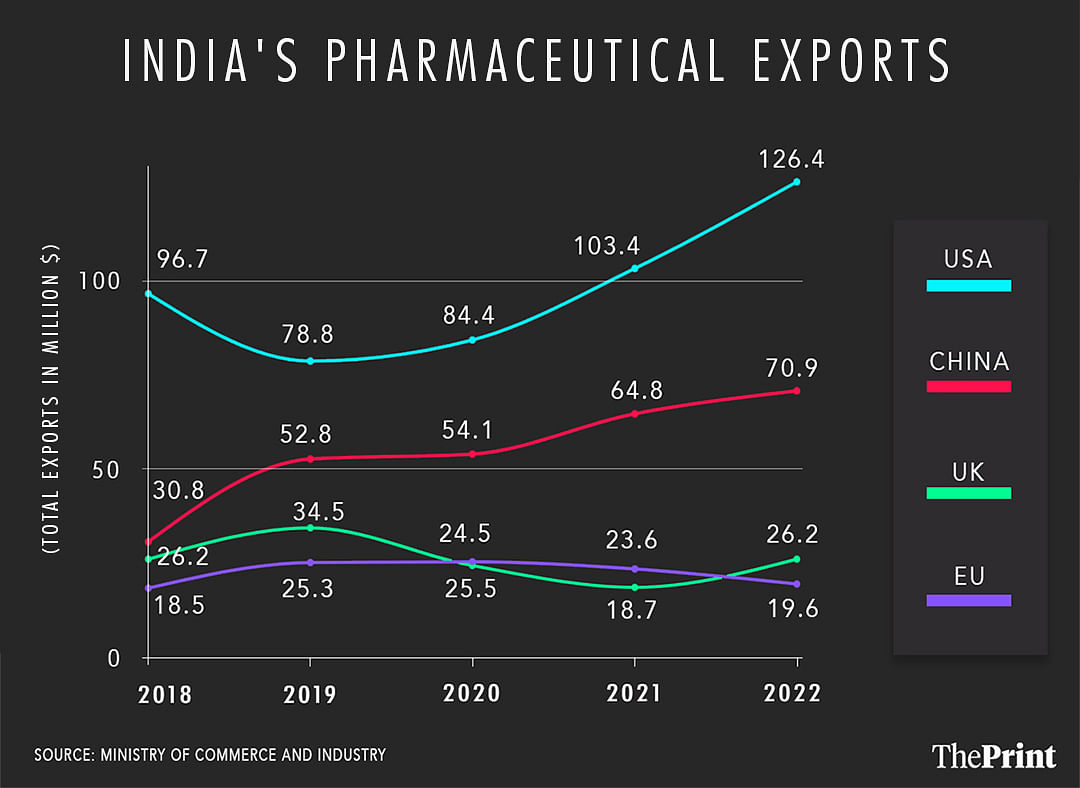
One of the largest suppliers of generic medicines, India accounts for about 20 per cent of their global exports, according to government data.
ThePrint reached the Indian Pharmaceutical Alliance through calls seeking its comments on the representation to the government. However, the association refused to comment on the matter.
ThePrint also reached the Department of Pharmaceuticals through email for a comment on the issue. The copy will be updated as and when a response is received.
Also Read: Intellectual property in the age of AI — why Delhi HC wants govt to relook Patents Act
‘Delay in launch of generics’
India currently does not have data exclusivity, but if introduced, it may ensure extended monopoly for originator companies in developing countries like India, though the patent may have expired in developed countries.
“This is because originators (innovators) launch their new drugs in low-priced countries years after their launch in the developed economies,” Indian drugmakers have said in the representation. “This would add to the period of monopoly for originators and delay the launch of generics.”
However, Suresh Pattathil, president of the Organisation of the Pharmaceutical Producers of India — a network of foreign drugmakers in India — said that the body believes that a robust & meaningful IP (Intellectual Property) environment is critical for not only boosting research and development in India but also addressing its unmet medical needs.
In March this year, over 200 health organisations and community groups had reportedly called on the UK’s chief negotiator for the UK-India FTA to resign amidst lack of transparency from the UK government.
The organisations had also cited the historic impact of Indian generic drugs, “including lowering HIV drug prices by 99 per cent from the year 2000 to today”.
In their representation to the central government, the Indian drugmakers have said the replacement of the patent regime in 1970 acted as a catalyst in achieving the title of the “pharmacy of the world” by giving a boost to the generic pharmaceutical industry.
India supplies medicines to over 200 countries, it added.
The concerns expressed by the drugmakers also resonated with some independent experts.
Ujjwal Kumar, former national consultant (Trade & Health) with the health ministry, who works with Delhi-based policy think tank CUTS International (Consumer Unity & Trust Society) told ThePrint: “Any changes in the Patents Act, which is WTO-TRIPS compliant, can have adverse impact on generic competition and access to medicines, not only within India and poor countries but also in developed nations like the US, UK, and EU.”
Promoting generic competition should be imperative for welfare states like India where out-of-pocket expenditure on healthcare is very high, he said, adding that if India decides to make changes in its patent regime, it must provide a credible public health justification, and ensure a public discussion on the same.
(Edited by Anumeha Saxena)
Also Read: Proposal to regulate prices of non-essential drugs on hold, RSS affiliate was among opponents



