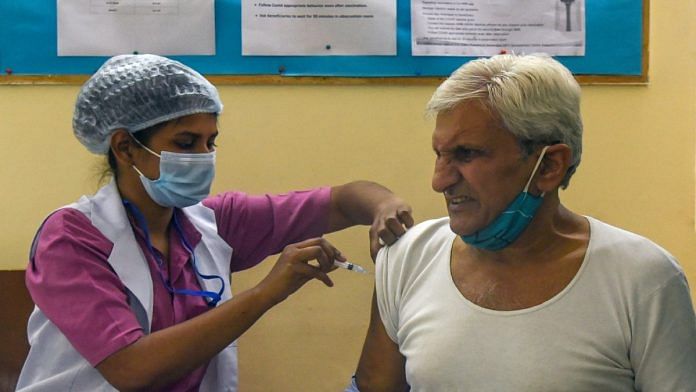
New Delhi: Two figures and a looming deadline have raised a question around India’s Covid vaccination drive.
India has administered roughly as many Covid vaccine doses within the country as it has sent for commercial exports. However, even as the earliest batches of the Covid vaccines are set to expire next month, the government refuses to allow domestic commercial sales.
Covishield and Covaxin, the two vaccines being used in India, were granted emergency use authorisation on 3 January. Since 16 January, when the Covid vaccination drive was rolled out in India, 3.48 crore doses have been administered in the country. Meanwhile, India has commercially exported 3.39 crore doses to countries around the world since the first of these batches went out to Morocco and Brazil on 22 January.
While the government has allowed the manufacturers of Covishield and Covaxin to individually negotiate the exports with different nations, they remain barred from selling the vaccines within the country.
The industry has repeatedly sent feelers to the government seeking permission for domestic sales, arguing that it will help them subsidise the government drive — vaccination is free at government hospitals, capped at private ones.
Speaking to ThePrint, an industry insider said the continued bar may prove detrimental to the health of the industry.
The government, however, has ruled out the possibility for now.
Asked about the resistance to vaccine sales, a senior health ministry official cited “restrictions under emergency use authorisation (EUA)”.
“Vaccines cannot be sold outside. They are under emergency use authorisation. Tell me which country in the world is selling vaccines?” the official said.
The Indian drug laws do not provide for EUA.
The New Drugs and Clinical Trial Rules do lay down an accelerated approval process under certain circumstances, but do not expressly debar sales.
In fact, they say: “After granting accelerated approval for such drug, the post-marketing trials shall be required to validate the anticipated clinical benefit.” This seems to suggest that drugs cleared via an accelerated approval process can be sold.
Also Read: Reasons behind Maharashtra’s disastrous Covid showing dragging the India story down
Subsidised
Of the two vaccines being used in India, Covaxin is an indigenous product developed by the Hyderabad-based Bharat Biotech. Covishield was developed by researchers at Oxford University and the British-Swedish firm AstraZeneca, and is being manufactured in the country by the Pune-based Serum Institute of India (SII).
The SII was the first company to start manufacturing vaccines in India, and has been stockpiling them since November 2020.
While it is possible that the earliest batches of the vaccine may have been used up in the past two months, government sources told ThePrint that, at a meeting a few weeks ago, the Union health ministry informed the cabinet secretary that the vaccines will start expiring from April.
The government is acquiring the vaccines from the two manufacturers at reduced prices — from the earlier cost of Rs 210 per dose, the cost has been renegotiated to a “significantly lower” level, as Union Health Secretary Rajesh Bhushan mentioned at last Thursday’s Covid briefing.
Sources say the present price per dose of vaccine is less than Rs 150.
It is for this reason, industry sources say, that manufacturers have been pushing for opening up of domestic vaccine sales — so that open sales can cross-subsidise the low-cost government procurement.
Private hospitals, which joined the vaccination drive with Phase 2 starting 1 March, have been allowed to charge recipients, but their procurement is also taking place through the respective state/UT governments, and the cost to patients is capped at Rs 250/shot.
Also Read: What is J&J Covid vaccine that India’s Bio E will make with US help, the challenges it faces
Vaccine Maitri
Most vaccine exports under ‘Vaccine Maitri’ — the Modi government’s international vaccine supply initiative — are commercial transactions
Of the total 586.4 lakh (5.86 crore) doses that India has sent abroad since the first grant tranches to Bhutan and the Maldives on 20 January, 339.67 lakh doses have been commercially exported to various nations. Another 165.48 lakh doses were given to Covax, an international initiative to make vaccines available for low- and middle-income countries. Just 81.25 lakh doses have gone out as grants to various countries.
Vaccine manufacturers have, for a while now, been seeking permission for sales to domestic private players. In an interview to The Economic Times this week, SII CEO Adar Poonawala said vaccine companies should be allowed to function without price restrictions.
Explaining their concerns, the industry source quoted above noted that “these companies have made investments early on, taking a risk”.
“They are not averse to giving the vaccines to the government at a discounted price. All they are asking for is for them to be allowed to sell outside and cross-subsidise the government procurement,” the source added.
“This is detrimental to the health of the industry. If the government, after so much talk about India supplying vaccines to the world, does not want vaccine companies to grow, we will have to live with that.”
Also Read: Ludhiana makes teachers, bankers, judges & journalists ‘frontline workers’ for vaccination



If 34.8 million are used in India and 33.9 million exported, this means 68.7 million are used. Do you mean to say manufacturers have stocks of more than 68.7 million doses before January that the extra dosage are expiring. Serum mentioned they have 50 million and 20 million was by Bhart Biotech, so this means that your article is misleading.