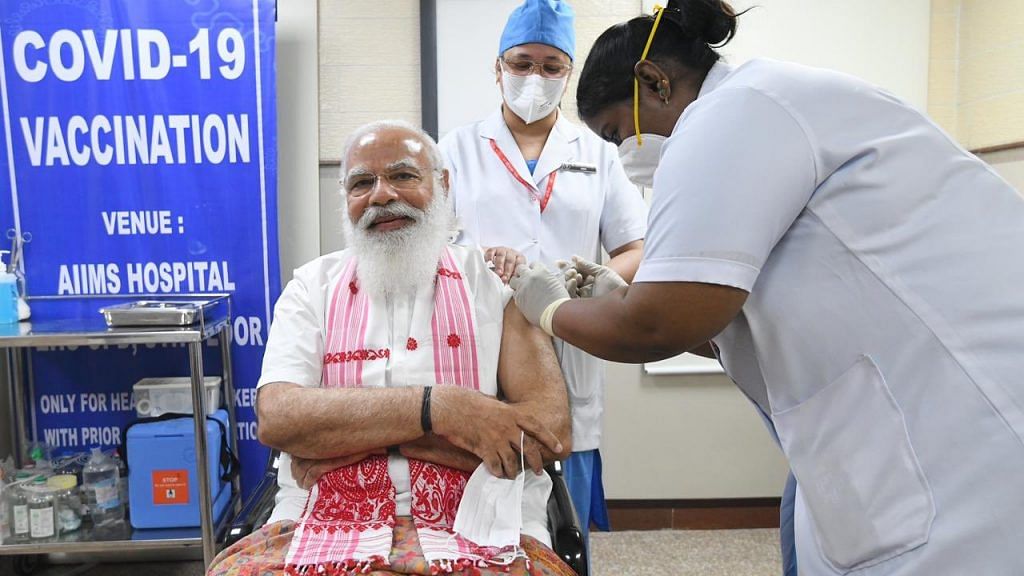
New Delhi: Prime Minister Narendra Modi took the first shot of Covaxin, the Covid-19 vaccine developed by Bharat Biotech and the Indian Council of Medical Research, becoming the first recipient of the shot as the second phase of Covid vaccination kicked off in India Monday.
The second phase will cover over 10 crore Indians in two priority groups — people above 60 years and those aged above 45 years who have comorbidities.
Modi visited AIIMS at 7 am where sister P. Niveda, who is from Puducherry, administered the Covaxin shot to him. The PM was sporting an Assamese gamucha.
A second nurse who was there to help was Rosamma Ali from Kerala.
The gamucha, said a statement from the Prime Minister’s Office, was “symbolic of blessings of women from Assam”.
Assam, Kerala and Puducherry are going to assembly elections this year, along with West Bengal and Tamil Nadu.
The PMO statement also said Modi decided to take the shot early morning, without any special route restrictions so people are not inconvenienced.
Soon after taking the vaccine, the PM posted a tweet commending doctors and scientists. ”Remarkable how our doctors and scientists have worked in quick time to strengthen the global fight against COVID-19,” he tweeted.
PM Modi also appealed to all those who are eligible to get themselves vaccinated.
“Together, let us make India COVID-19 free,” he posted.
Took my first dose of the COVID-19 vaccine at AIIMS.
Remarkable how our doctors and scientists have worked in quick time to strengthen the global fight against COVID-19.
I appeal to all those who are eligible to take the vaccine. Together, let us make India COVID-19 free! pic.twitter.com/5z5cvAoMrv
— Narendra Modi (@narendramodi) March 1, 2021
Also read: From vaccine hesitancy to vaccine eagerness. In phase II, Modi govt has new challenges
Nearly 1.1 crore vaccinated in first phase
On 24 February, India announced that it will from 1 March onwards start administering Covid-19 vaccines to people above 60 years and those aged above 45 years who have comorbidities.
In the first phase that kicked off on 16 January, vaccinations were made available for health workers across India, with frontline workers allowed access earlier this month.
A total of 1,07,67,000 people had received the first shot of the vaccines’ two-dose regimen by Wednesday. Of these, 14 lakh have received the second dose, which is administered after a four-week gap.
Health workers, frontline workers, the elderly and those with comorbidities are the four priority groups identified by the Modi government for the Covid vaccination drive, since they are believed to be most exposed or vulnerable to infection.
Vaccination was free for the health and frontline workers.
The government has decided to offer free vaccines to all those who visit the 10,000 government medical facilities around India.
During the second phase, private hospitals have been allowed to administer the vaccine.
Those going to private hospitals, however, will have to pay for the doses. The authorised hospitals can charge up to Rs 250 for every dose.
“States have been explained that the private hospitals functioning as CVCs (Covid Vaccination Centres) can charge subject to a ceiling of Rs 250 per person per dose along with the electronic and financial management mechanism in this regard,” the health ministry said in a statement Saturday.
The Indian vaccination programme currently comprises two vaccines. Covishield, developed by researchers at the Oxford University and British-Swedish pharma firm AstraZeneca, is being manufactured in India by the Pune-based Serum Institute of India (SII). Home-grown Covaxin is developed by Hyderabad-based Bharat Biotech.
The central government has committed to buying approximately 6 crore doses of vaccines from SII and Bharat Biotech, of which a little over 1.5 crore have already been delivered.
The vaccines are usable for six months and the first lot will expire in April.
Also read: Why an efficient vaccine is not the same thing as a safe vaccine