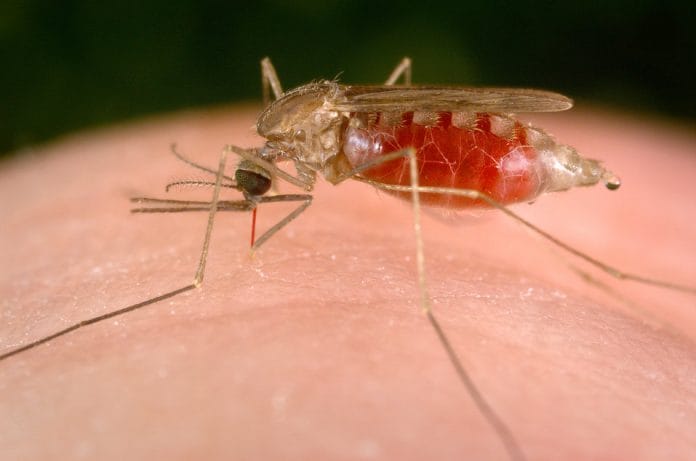
Bengaluru: A genetically-modified mosquito killer has helped decimate 99 per cent of malaria-carriers within 45 days in a study that promises a powerful prevention technique against the disease.
The study focuses on a fungus, Metarhizium pingshaense, that is known to infect the female Anopheles mosquito, the carriers of malaria, in the wild.
Researchers from the University of Maryland (UMD), US, and Burkina Faso’s IRSS (Institut de Recherche en Sciences de la Santé) research institute modified the fungus with a spider venom known to kill mosquitoes.
The result, according to researchers, was a killer fatal to mosquitoes alone and safe for other insects like bees. The findings were published in the journal Science on 31 May.
“No transgenic malaria control has come this far down the road toward actual field testing,” said Brian Lovett, a graduate student in UMD’s Department of Entomology and lead author of the paper, in an accompanying statement.
“This paper marks a big step and sets a precedent for this and other transgenic methods to move forward.”
Also read: World’s first-ever malaria vaccine has just been rolled out in Africa
Method and results
Metarhizium pingshaense is already used as an insecticide, sprayed directly on crops across the world as it affects multiple insects naturally. Since it infects mosquitoes in the wild, it offered researchers an easy carrier to target the Anopheles.
The researchers then figured out how the funnel-web spider, native to the Australian Blue Mountains, produces its venom: The venom contains a toxin called ‘Hybrid’, which kills mosquitoes and other insects.
The next step saw the researchers genetically re-programme the DNA of the fungus in a manner that would allow it to produce Hybrid after it was lodged in an Anopheles host. This was done by a standard genetic modification technique, where a bacteria’s DNA is transferred into a fungus.
The DNA transferred in the study carried the blueprint for producing Hybrid, with the researchers building a trigger or ‘control switch’ to ensure Hybrid is only produced when the fungus is inside a mosquito.
The fungus was first tested in labs, at both Maryland as well as Burkina Faso, to ensure it affects only mosquitoes and is harmless to other insects like bees. It was then released outside the lab, into a simulated, controlled setting that mirrored a village, measuring 6,550 square feet in area and enclosed by screens.
The test village, called MosquitoSphere, had huts, plants, mosquito-breeding pools, and food sources, divided into three separate chambers.
In one hut in each of the chambers, the researchers hung black cotton sheets coated with sesame oil. In one of the chambers, the researchers mixed the transgenic fungus into the oil. In the second, the oil contained the natural version of the fungus, while the coating in the third chamber was plain oil.
In each chamber, 1,000 adult male and 500 adult female mosquitoes were introduced at the breeding stage.
According to the researchers, in 45 days spanning two generations of the mosquitoes, the fungus caused the collapse of the Anopheles population to unsustainable levels in the chamber that contained the transgenic fungus. There were only 13 adult mosquitoes left by the time the study ended. In contrast, the second and third chambers were found to have 455 and 1,396 adults, respectively.
The experiment was repeated multiple times with very similar results, the researchers said.
It was also found that while uninfected females laid 139 eggs that yielded 74 adults in 45 days, the ones infected with the transgenic fungus laid only 26 eggs, of which about three developed into adults.
Malaria in Africa
Malaria has existed in the world for several million years, with the oldest record dating back 30 million years. The disease infects over 20 crore people every year, with lakhs of patients dying.
As many as 88 per cent of the infections and 90 per cent of the deaths occur in Africa alone.
To combat this, African countries like Burkina Faso, Mali, and Uganda are adopting urgent and cutting-edge measures.
One such step is genetically-modified mosquitoes, which work in two ways. One of the methods involves the transmission of a sterility gene through males so that new male offspring are all sterile and hence unable to reproduce.
Carrying out a genetic modification to affect future populations is called ‘gene drive’.
The other method is to infect the females, as these are the only ones capable of transmitting malaria, as done in the new study.
Also read: Latest tool in fight against malaria: Dogs that can sniff out malaria from our socks
Since the malaria-transmitting mosquito, the anopheles gambiae, is not a ‘keystone’ species, its complete extinction will most likely not affect the ecosystem at all.
“We demonstrated that the efficacy of the transgenic fungi is so much better than the wild type that it justifies continued development,” said Raymond St. Leger, a professor of entomology at UMD and co-author of the study, in the statement.