The drug companies are eager to study ‘essential fructosuria’, a genetic mutation which could unlock potential treatments for weight-related ailments.
For a lucky few people in the world, weight will never be a source of worry.
People who have a rare genetic mutation called essential fructosuria lack the primary enzyme needed to metabolize fructose, a natural sugar found in honey, fruits and vegetables. No ill consequences have been linked to the defect, save for an aversion to sweets, and it appears to have a significant benefit: Those who have it seem to be at little risk of obesity, type 2 diabetes or serious liver ailments.
“When I started contacting people who were taking care of these patients, there was literally no one — I couldn’t find anyone — who was fat or had type 2 diabetes,” said Richard Johnson, a pioneering metabolic-disease scientist at the University of Denver.
Researchers looking to understand weight-related ailments — and drug companies seeking new medicines to treat the vast population of people who suffer from them — are eager to study essential fructosuria. The mutation could unlock potential treatments after years of failures to find safe and reliable therapies.
The problem is, it is almost impossible to find people who have it.
The chance that someone has essential fructosuria is 1 in 130,000 — longer even than the odds of dying in a severe storm. The last case report in medical literature is from 1998, according to Mark Herman, an assistant professor at researcher at Duke University School of Medicine. On top of that, people with essential fructosuria tend to be healthy. When patients are found, they are often stumbled on by chance, surfacing only when a carrier pops up at a doctor’s office for some other reason.
Essential fructosuria is so uncommon that even Pfizer Inc., the largest U.S. drugmaker with $53 billion in 2017 sales and products in more than 125 countries, has been unable to track down a single carrier. That hasn’t stopped the company from trying to develop a drug modeled on the mutation that could reverse or prevent progression of the liver disease nonalcoholic steatohepatitis, known as NASH — and perhaps one day treat more common conditions.
“We hope long-term we will actually prevent insulin resistance and obesity,” said Pfizer Senior Vice President Morrie Birnbaum, who leads the New York company’s internal medicine research division.
The human body doesn’t make a lot of fructose on its own. It gets it from foods and sugary drinks. Consuming too much fructose is associated with liver disease and Type 2 diabetes and is usually responsible for a condition called fatty liver, which can lead to NASH. Inhibiting metabolism by the fructose enzyme, called fructokinase, has been shown to reduce the risk of developing those diseases.
The idea of curbing fructose metabolism to prevent disease largely began with Johnson, the University of Denver scientist. His pivotal finding came when he discovered that mice without the fructokinase enzyme gained less excess fat and weight and had lower blood glucose and insulin levels than those with the enzyme when given a high-fructose diet.
Studies by scientists Kimber Stanhope and Peter Havel, of the University of California at Davis, also showed that fructose was associated with weight gain and increased the risk factors of metabolic syndrome in humans. Until their research was published in 2009 in the Journal of Clinical Investigation, many scientists weren’t convinced that fructose was problematic. Some believed it was helpful, as it didn’t boost blood-glucose levels, a factor in diabetes, according to Stanhope.
“At this point, inhibiting this enzyme is the best pharmaceutical solution to this high sugar diet,” Stanhope said. “When you have fructokinase, it’s such a strong enzyme that it pulls 90 percent of the fructose in a big gulp into the liver.”
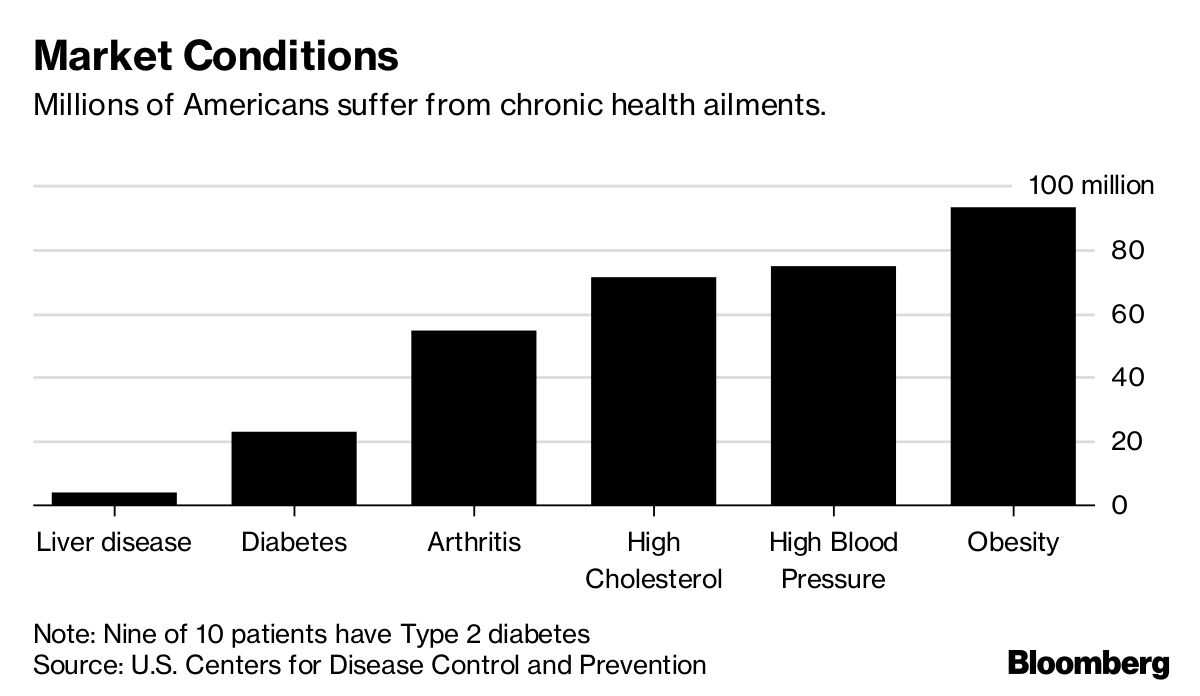
Finding an obesity drug has been a longstanding goal for the world’s biggest pharmaceutical companies — even though the American Medical Association didn’t consider it a disease until 2013. Two-thirds of Americans are considered overweight or obese. But treating weight problems has proven to be a perplexing puzzle of diet, genetics, behavior and environmental factors. Several therapies have been brought to market only to be pulled from the shelves later because of safety issues or slow sales.
Johnson & Johnson tried more than a decade ago to bring a fructose metabolism inhibitor to market after chemist Bruce Maryanoff, who discovered the blockbuster anti-epilepsy treatment Topamax, developed several of them. However, the drug failed before ever reaching a clinical trial, and J&J didn’t think funding more research would yield a commercial success, the now-retired Maryanoff said in an interview.
A JNJ spokeswoman said the company doesn’t currently have any fructose-inhibitor programs.
J&J and Maryanoff’s published data, along with the research being done at the University of Denver by Richard Johnson, caught the attention of scientists at Pfizer in 2011. Maryanoff’s team at J&J was the first to use the basic chemical structure of fructokinase in drug discovery. Pfizer’s scientists saw something to build and improve upon.
“We saw their leads and we thought we could do better. I knew we could do better,” said Thomas V. Magee, one of the co-inventors of Pfizer’s compound, which is called PF-06835919. “I said to my boss, ‘I want to work on this.”’
Initially, Pfizer’s outside advisory board rejected the pursuit of a fructose inhibitor on the grounds that it didn’t seem possible to stop the metabolism and prevent disease. There were also concerns over what happens to excess fructose that doesn’t leave the body through urination. Ultimately, Pfizer was persuaded that people with essential fructosuria have high sugar levels in their blood without problems.
After screening thousands of compounds, Pfizer launched its first preclinical study in 2013, followed by human trials three years later. A mid-stage study of 53 patients recently wrapped up.
One challenge, Birnbaum said, is that no one knows how many people with fat in their liver got it by eating a lot of fructose, and how many have it simply as a result of their body chemistry. Since Pfizer believes the drug is likely to work best in people who consume high amounts of fructose, but also be effective in most people with fatty liver and NASH, knowing how much unnatural fructose is consumed is crucial.
Pfizer’s trial will provide the answer, Birnbaum said.
If the treatment works, it would be taken daily to prevent the buildup of fat cells in the liver. NASH’s effects include inflammation, scarring and metabolic changes. Up to 12 percent of Americans are estimated to have it. Not everyone with fatty liver ends up coming down with NASH, but Birnbaum compared the idea of reducing fatty liver to help prevent NASH to reducing cholesterol to help prevent heart disease. A drug aimed at controlling a common condition in a large number of people in that manner could be lucrative: The blockbuster cholesterol fighter Lipitor once accounted for a quarter of Pfizer’s revenue.
Pfizer’s focus on fructose is unique among companies developing medicines for asymptomatic NASH, which is projected to be the top reason for liver transplants by 2020. Intercept Pharmaceuticals Inc., Madrigal Pharmaceuticals Inc. and Gilead Sciences Inc. have NASH drugs in late-stage studies, but they are targeting the sickest patients. The NASH drug market is expected to be worth about $40 billion by 2025.
Meanwhile, Johnson, 64, is developing his own drugs, which he hopes to sell some day. But he’s realistic about beating a giant like Pfizer from a 10-person research lab on a suburban college campus.
“They should be viewed as competing with us, and probably beating us,” he said with a chuckle. But Johnson said he wants Pfizer to succeed, as it would validate much of his research. “What we’re looking at is a medicine that prevents the development of obesity, prevents diabetes. My belief is this will have a major clinical impact.” – Bloomberg






