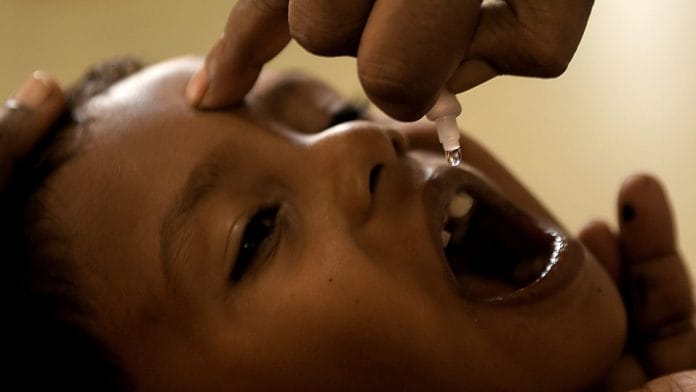
Bio-Med is accused of releasing polio vaccines contaminated with the Type-2 virus administered to 30 lakh children.
New Delhi: The Ghaziabad-based vaccine manufacturer accused of releasing contaminated polio vaccines wants a re-test of the batches that triggered a major health scare in September.
Bio-Med Pvt Ltd has challenged the investigation report of the Central Drugs Standard Control Organisation (CDSCO), the country’s apex drug regulation body under the ministry of health and family welfare, which contained the damning findings.
The report, at the heart of the entire controversy, alleged that some batches of the oral vaccines for polio manufactured by the firm contained traces of the Type-2 virus, which was declared eliminated years ago.
It said as many as 30 lakh children may have been infected in two states, triggering an alarm even as the government said there was no cause for worry.
The octogenarian managing director and founder of Bio-Med was then arrested.
Bio-Med has questioned the findings in a letter to the CDSCO, seen by ThePrint, and asked the drug regulator to re-test the samples. “Bio-Med has challenged the findings of our investigations and we will be sending their samples for re-testing,” said a senior official at the CDSCO.
According to the Drugs and Cosmetics Act, if a drug fails a quality test or is deemed to be “not of standard quality”, the manufacturer can challenge the investigation, but within 28 days of receiving the report. Though the exact date of the letter could not be affirmed, Bio-Med kept to the deadline.
The government will now send the samples for a re-test to the Central Drugs Laboratory (CDL), Kasauli, the same facility that made the initial findings. The conclusion drawn in the second inquiry will be considered final.
Bio-Med did not respond to an email from ThePrint seeking comment.
Also read: The detailed story of how India won the battle against poliovirus
The controversy took drug regulators to Indonesia
Indian drug sleuths took the investigation international as they sought to find the source of the polio Type-2 virus. An examination of the Bio-Med facility had earlier led investigators to conclude that the contamination was unlikely to have taken place there, even though they did reportedly discover some violations of “good manufacturing practices”.
On 29 October, they flew to Indonesia to investigate a firm, Bio Farma, from which Bio-Med sourced raw material for their polio vaccine. “Before taking any further action [against Bio-Med], we went ahead to check the quality of the raw material used,” said the CDSCO official ThePrint spoke to.
However, the team came back with “no major” findings. “The drug inspectors are yet to file the official report,” said an official from the health ministry. “However, they have not found any evidence that could prove the vaccine’s raw material was contaminated,” the official added.
Earlier, in an emailed response to ThePrint, Bio Farma had denied its samples were contaminated.
Meanwhile, sources said, the pharma sleuths will pursue Bio-Med for the lapses allegedly discovered in their manufacturing process.
The CDSCO is also said to be considering moving the Supreme Court against the decision of a Ghaziabad court to grant bail to the firm’s managing director, the 81-year-old Dr S.P. Garg. He was released on bail after 14 days in judicial custody.
To this end, the regulator has asked the law ministry for its opinion. “We have no powers to arrest someone but we are certain about the foul play at the firm,” said a member of the team that examined the Bio-Med facility. “We have checked the premises of Bio-Med and collected several pieces of evidence.”
Also read: Govt moves to contain panic after 30 lakh kids get ‘eradicated’ polio strain with vaccine