New Delhi: Clean hands have become the first and final frontier against contracting the coronavirus, and with countries running short on hand sanitisers and face masks, experts say washing hands with soap and water is the best way to avoid the spread of the infection.
ThePrint explains exactly how the humble soap can be an effective defence mechanism against the COVID-19 pandemic.
How does the coronavirus spread?
Any type of virus remains inactive until it actually invades living cells. When a person coughs or sneezes, tiny droplets carrying viruses can fly a distance of up to 2 metres.
While these droplets end up on surfaces and dry out quickly, the viruses can remain for a long time, such as two or three days on metals. When humans touch such surfaces, the virus sticks to the skin of our hands. When a person touches his/her eyes, nose or mouth, it could lead to an infection.
Also read: Coronavirus is not an evil Chinese bioweapon meant to inflict terror. Here’s why
Structure of the coronavirus
Simply put, the novel coronavirus has three essential components.
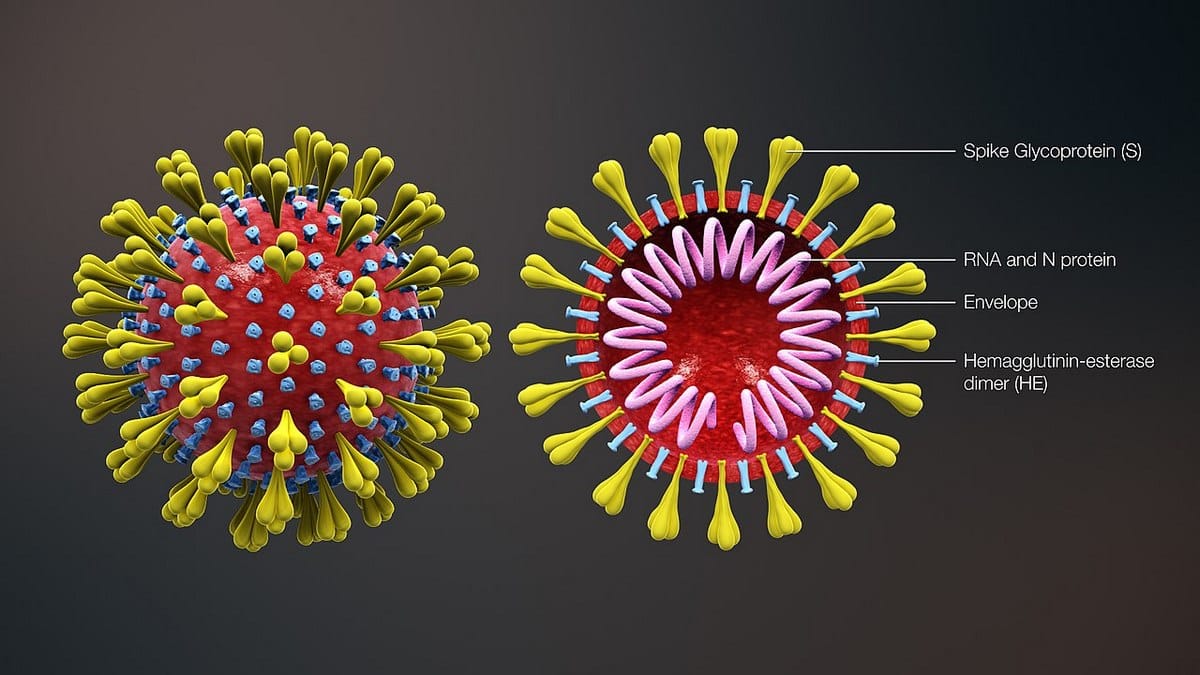
Proteins on the surface help the virus latch onto and infect living cells. The second component is ribonucleic acid (RNA), the genetic building block that replicates only when the virus is inside a living cell. The third component is a lipid layer — essentially a fatty envelope — that holds the virus.
This envelope is what appears to be the Achilles’ heel of the virus, as breaking this layer causes the whole virus to fall apart.
Also read: How coronavirus spread in India — 39 of the first 50 patients came from Italy, Iran, China
How soap works on the virus
Usually, fatty particles — such as oil and grease — do not mix with water, making it difficult to clean dirty hands or any other surface with just water. This is where the chemistry of soaps comes in.
Soaps are made of a long chain of hydrogen, oxygen and carbon atoms. Each hydrocarbon chain has a ‘salt’ at one end — usually consisting of a sodium or potassium atom.
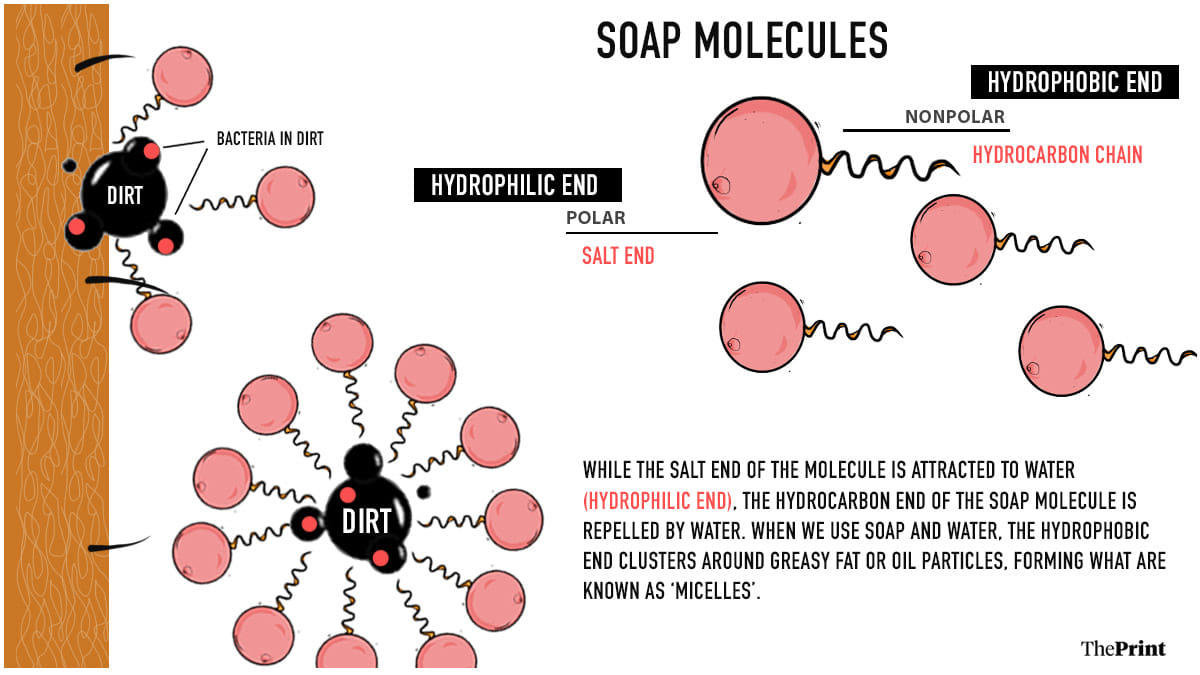
The hydrocarbon end of the soap molecule is repelled by water. When soap and water are used together, this end of the soap molecule sticks to the oil or fat particles, while the ‘salt’ end sticks out.
The soap molecules arrange themselves into spherical clusters around small oil particles. These structures, known as micelles, get easily dispersed in water.
This mechanism is what helps soap break down the weak, lipid outer layer of the coronavirus, which holds all of it together.
Soap more effective than hand sanitiser
While alcohol — the primary ingredient in hand sanitisers — can also break down the lipid layer of the coronavirus, it does not bond with the virus material as quickly as soap.
Hand sanitisers are a good option when you do not have immediate access to water and soap, but the latter works much better in breaking down the virus.
Also read: Fever, cough, fear or myths — Indians are turning to e-doctors for all coronavirus ‘cures’






