New Delhi: A group of researchers from around the world has alleged data discrepancies in a study published in The Lancet last week that reported the Russian coronavirus vaccine candidate, Sputnik V, had been successful in producing an immune response in Covid-19 patients.
Originally written by Enrico Bucci, a biochemistry and microbiology expert at Temple University, US, Monday, the letter has since received the backing of at least 23 other scientists who have lent their support as co-signatories.
The Lancet study, published on 4 September, reported that the two-part Sputnik V was able to trigger both an antibody as well as a T-cell immune response in recipients, without producing any serious adverse effects. The vaccine was developed by the government-backed Gamaleya Research Institute of Epidemiology and Microbiology (GRIEM), and the project was funded by the government-backed Russian Direct Investment Fund (RDIF).
In their open letter to the researchers and Lancet editor Richard Horton, Bucci and others have pointed out that a number of graphs used to present the study results had unexplained repetitive patterns.
“During the current pandemic, the public extreme interest and expectations for an effective vaccine are fully understandable. However, the very same reasons should motivate the scientific community to pay even more attention to the scientific evidence and the underlying data, and it is thus of utmost importance that they are fully available for close scrutiny,” Bucci wrote in the letter.
They have sought the numerical data for all the experiments to evaluate the present study.
An email sent to the corresponding authors of The Lancet study had not elicited any response by the time of publishing.
The press communication division of The Lancet said in an emailed statement that it is “aware of the open letter on the Russia vaccine trial”. “We encourage scientific debate on papers we have published… We have shared the letter directly with the authors and encouraged them to engage in the scientific discussion,” it added.
Also Read: The Lancet, world’s most credible medical journal whose trust has been hit by HCQ scandal
The ‘discrepancies’
Among the graphs pointed out by the authors is one (labelled as ‘figure 2’) that seeks to report the levels of different antibodies at different points in time, for all the patient groups (challenged with the two different formulations of the vaccine).
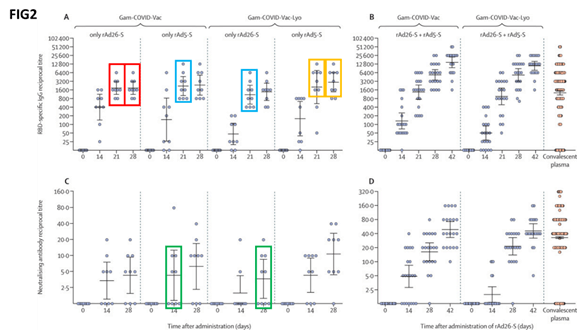
“In the red boxes, 9 out of 9 volunteers challenged with rAd26-S appear to have identical antibody titres at 21 and 28 days; this is also true for 7 out of 9 volunteers challenged with rAd5-S (yellow boxes),” Bucci wrote in the letter.
“Furthermore, in the cyan boxes, we can see all the experimental points differing for a constant value in two completely unrelated experiments,” he added.
Eight out of nine experimental points are identical among other two completely unrelated volunteer groups as highlighted by the green boxes, the researchers said.
Another graph labelled ‘figure 3’ shows the cellular response to the different formulations. Again, the researchers claimed to have identified repeated patterns by boxing them with the same colours.
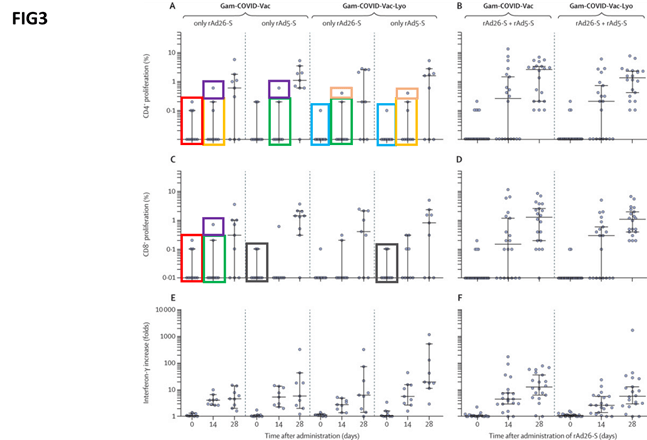
They point out that, given that cellular responses are extremely variable and differ from person to person, a pattern emerging in these data points among different experiments is unlikely.
Repetition has also been alleged with respect to ‘figure 4’ in the study, which is intended to show the neutralising antibody formation.
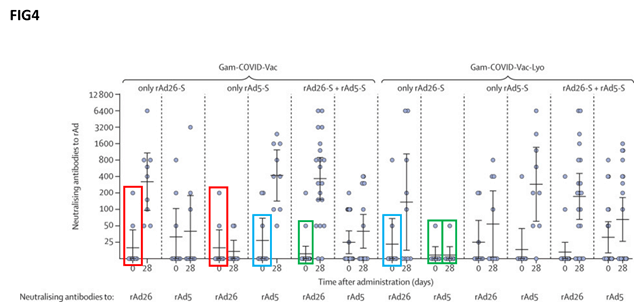
“It seems to us that on the ground of simple probabilistic evaluations the fact of observing so many data points preserved among different experiments is highly unlikely,” the researchers wrote in the letter.
Shahid Jameel, an Indian virologist and chief executive officer of Wellcome Trust DBT India Alliance, which funds research in health and biomedical sciences, said the points raised by Bucci are valid.
“Without access to the actual data, the points and patterns on the graphs look too similar at multiple places. This raises concern about the data, and, therefore, the way this vaccine was tested,” Jameel said.
With only 40 volunteers, he added, the trials are “underpowered”. “This raises genuine and serious concerns about the phase 2 data,” he said.
“Journals depend upon peer review to accept or reject papers. This should have been picked up by the reviewers. However, a prestigious journal such as Lancet cannot absolve itself of the responsibility,” Jameel added.
Also Read: Russia in talks with India for ‘Sputnik V tech transfer’ to boost production, exports
‘Research groups under pressure’
This is not the first time during the Covid-19 pandemic that The Lancet, one of the world’s most prominent medical journals, has come under the scanner for research related to the pandemic.
In June, the journal had to retract a study that sought to link the anti-malarial drug hydroxychloroquine (HCQ) — which was cited as a promising treatment and preventive in the earlier stages of the pandemic — to higher risk of death and irregular heart rhythms in Covid-19 patients.
The study had even prompted the World Health Organization (WHO) to pause the HCQ arm of its global ‘Solidarity trial’ to find an effective treatment for Covid-19. However, the study soon came into question for dodgy data.
Speaking to ThePrint, some researchers noted that fresh The Lancet study does not present the original data from which the graphs in question were made.
“Having access to this original data should help clarify these discrepancies, said Gautam Menon, an infectious disease expert who teaches at Ashoka University.
Satyajit Rath, a researcher at IISER Pune, sought to link data-related discrepancies to the pressure scientists are under in light of the pandemic.
“I am not surprised that discrepancies, some more minor than others, will keep getting found in most Covid-19-related publications, given the pressure that all research groups in the area will be working under,” he said.
“Both journals and reviewers will be under pressure to make rapid decisions, too. So, it is not surprising, again, that discrepancies slip by. Given the intensive scrutiny that most, if not all, such publications (even preprints) are being subjected to, I doubt that the practical consequences of such errors, if any, will be particularly major,” Rath added.
This report has been updated with The Lancet‘s statement on the matter
Also Read: ‘Star’ cardiologist Mehra & data doctor Desai — story of Indian experts behind HCQ scandal







Expect similar things from our ICMR scientists when they publish the results of current study. Normally, they are not involved in conducting the study or data collection or data analysis. But given the current environment, who would not want to take centre stage and talk about vaccine clinical trial data.