New Delhi: India will soon begin clinical trials of a plasma treatment for critical Covid-19 patients, according to the Indian Council of Medical Research (ICMR), the country’s apex body in the field.
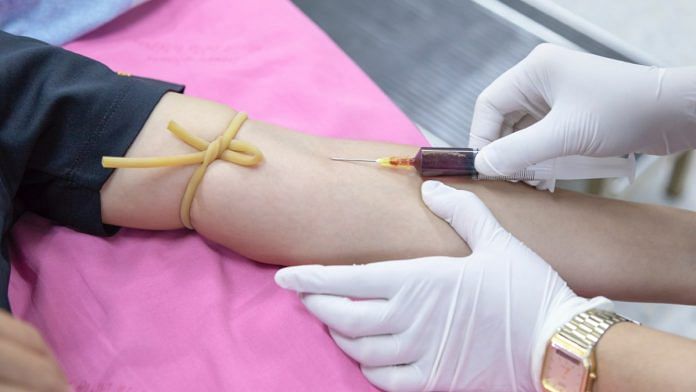
The treatment includes injecting patients with plasma from people who have recovered from the infection, and whose bodies have therefore generated the antibodies required to fight the virus.
This is an important development as there is no tried and tested anti-viral drug or vaccine against the novel coronavirus yet.
Also read: What is rapid antibody test that India has cleared for Covid-19 and how it will help
The plasma in the therapy
Called convalescent plasma therapy, it works on the principle that the antibodies of a recovered person can help the recovery of a sick patient. The antibodies are present in the plasma, the yellowish liquid part of the blood.
People who earlier tested positive, have since recovered and show no signs of the infection for 2-3 weeks will be selected as donors.
Speaking at the daily press conference held by the health ministry, Manoj Murhekar, director of the Indian Institute of Epidemiology said Thursday that the ICMR is in the process of finalising the protocol for the clinical trial, which will begin after final approval from the Drugs Controller General of India (DCGI).
“This will not be for mild patients, but (for) those who are on ventilators and under clinical trial mode, before being recommended for all patients,” said Murhekar, who added that the therapy was found to be successful in other countries in limited clinical trial settings.
Kerala has already received ICMR approval to begin the trial, he said.
Other centres that want to try the therapy will need the DCGI’s approval.
Also read: These 5 anti-viral drugs and therapies could help treat COVID-19
Kerala looks to build plasma bank
A member of Kerala’s expert committee, who did not wish to be named, said the approval allows the state to begin the process of collection of plasma from patients who have recovered.
The convalescent plasma project in Kerala is being led by intensivist Dr Anoop Kumar of Baby Memorial Hospital in Kozhikode. An intensivist is a board-certified physician who provides special care for critically-ill patients.
Kumar is part of the state’s expert group, and had earlier worked on the Nipah virus outbreak.
Kumar has put together a team of experts from other health institutes who are in the process of finalising the procedure for identifying possible donors, criteria for the levels of antibodies, etc., based on global studies.
“The current effort is not to transfuse the plasma in patients but to begin the process of drawing and storing the plasma from donors to keep it ready when the time comes,” said the expert committee member.
Also read: $50mn for hydroxychloroquine clinical trial to new testing tech — top Covid-19 research news
Past trials
Plasma therapy was successfully employed to tackle other epidemics such as SARS, H1N1 and MERS. It was also used to deal with cases of measles and mumps.
Early Covid-19 trials have been encouraging. In one such trial, 10 critically-ill patients from Wuhan, the initial epicentre of the outbreak, were transfused with one dose of 200 ml of convalescent plasma. The patients showed improvement in clinical symptoms in three days, while the levels of coronavirus in the blood disappeared in seven days with no side-effects.
Researchers called convalescent plasma therapy a “promising rescue option” for severe Covid-19 patients, but added that large randomised control trials are needed.
The study appeared on 6 April in the peer-reviewed journal Proceedings of National Academy of Sciences of the United States of America.
Last month, the Food and Drug Administration in the US approved the therapy in patients as an experimental treatment for critically-ill patients who had no other treatment options.
In the US, 57 institutions in 46 states have come together to form the National Covid-19 Convalescent Plasma Project to investigate the use of convalescent plasma therapy.
Also read: ‘Test more, prepare post-lockdown plan’ — over 800 scientists appeal to Modi govt



Good information.
Patient to Permit in Writing for Clinical Trial on him or her? This Question Needs Answer. By the Union Govt.
Very good information .
Excellent move.
Kerala I am proud of you.
Your progress, your professionalism, your dedication to make life better. your focus for humanity is unlike SOME WHO ALWAYS TOUT FOR NO ACHIEVEMENT in reality.