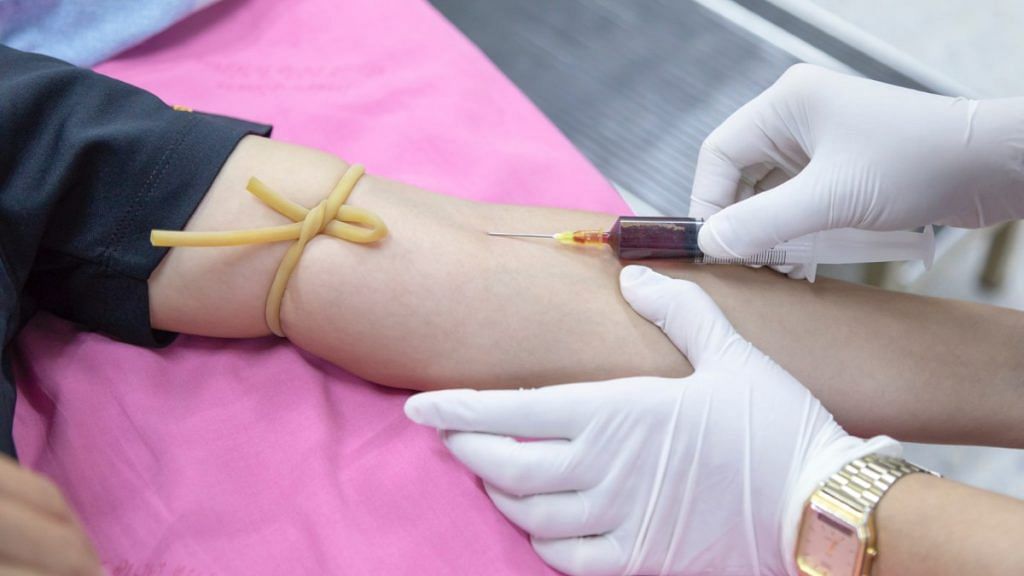
New Delhi: India’s apex health research body, the Indian Council of Medical Research (ICMR), is hoping to begin two clinical trials of plasma therapy in the next two weeks for the treatment of critically ill Covid-19 patients.
On 12 April, the ICMR uploaded proposals, inviting health institutions, hospitals to participate in two randomised controlled trials — one for convalescent plasma therapy and another for plasma exchange therapy.
Convalescent plasma therapy involves injecting patients with plasma from people who have recovered from the infection, and whose bodies have, therefore, generated the antibodies required to fight the virus.
Plasma exchange therapy includes removal of plasma from a sick patient and replacing it with plasma from a healthy donor.
The ICMR, however, makes it clear that it does not recommend the plasma therapies as treatment outside clinical trial settings.
In the absence of a vaccine or a specific drug, plasma therapies have been tested globally, albeit in limited numbers, as a treatment for patients who show severe complications due to coronavirus.
About 5-10 per cent Covid-19 patients in the world have respiratory, kidney and other complications, and require ventilation and need to be treated in intensive care units.
Also read: These 5 anti-viral drugs and therapies could help treat COVID-19
Enrollment of patients to start in next 1-2 weeks
The clinical trials are awaiting nod from the Drug Controller General of India and the ICMR’s own ethics board. The trials will then be registered in the clinical trial registry in the next 2-3 days, said a researcher involved in the initiative, who did not want to be named.
“We can begin enrolling patients in the next one or two weeks,” said the researcher, who expects more than 20 institutes to participate.
Plasma samples will undergo additional testing at the National Institute of Virology, Pune, to ascertain their quality.
Institutions that have the equipment and infrastructure can participate in the clinical trials after getting the required approvals and clearances.
Each centre will have its own principal investigator who will implement the trials that will be coordinated by the ICMR, said the researcher.
The ICMR’s trials are randomised control trials, meaning there will be two groups — the conventional therapy (using medicines that are currently being used to treat Covid-19) and the new plasma therapy.
The results of the trials may be known in the next two or three months, the researcher added.
Convalescent plasma therapy
The ICMR’s own trial comes days after Kerala received its approval from the health research body to begin the convalescent therapy trial involving various centres in the state.
Confirmed Covid-19 patients, who have recovered and show no symptoms for two weeks and more, are selected as donors for convalescent therapy.
As of Tuesday evening, India has 8,988 active cases of coronavirus, with 339 deaths. As many as 1,035 patients have cured, and they could possibly be donors for the plasma therapy.
Last month, the Food and Drug Administration in the US approved the plasma therapy as an experimental treatment for critically ill patients who had no other treatment options.
Also read: What is rapid antibody test that India has cleared for Covid-19 and how it will help