New Delhi: For blood cancer patients in India suffering from an advanced or relapsed form of the disease, another “living drug” has been cleared, nearly a year after the country’s apex drug regulator approved the first homegrown cutting edge immunotherapy, CAR-T therapy.
A “living drug” is a therapy which involves removing and modifying a patient’s cells, then infusing them back into the patient. They are also known as cellular therapies.
The Central Drugs Standard Control Organisation (CDSCO) has approved Chimeric Antigen Receptor or CAR T-cell, named Qartemi by Immuneel Therapeutics, for adult B-cell Non-Hodgkin Lymphoma (B-NHL), a common and aggressive type of blood cancer.
The company is a cell-and-gene therapy start-up backed by Biocon founder Kiran Mazumdar Shaw and US-based oncologist and author Dr Siddhartha Mukherjee.
Called Qartemi India’s “first international CAR T-cell therapy”, the product has been licensed from Hospital Clínic de Barcelona (HCB), a globally renowned institution for cell therapy innovation.
In response to a query by ThePrint, Immuneel said the therapy would cost about Rs 35 to 50 lakh, but still a fraction of what the treatment costs in the US.
As part of clinical trials in the country – named IMAGINE — the therapy was tested in blood cancer patients across Narayana Hospital in Bengaluru, Apollo Cancer Hospital in Chennai and Postgraduate Institute of Medical Education and Research (PGIMER) in Chandigarh.
Immuneel said that in the Phase 2 trial, the therapy achieved 83.3 percent ORR or overall response rate (the measurement of partial or full response in patients following a treatment) – “setting a new benchmark for CAR-T therapy in India”.
India faces a rising burden of blood cancers, with around 120,000 new cases and over 70,000 deaths annually from leukemia, lymphoma, and multiple myeloma.
Kiran Mazumdar-Shaw, board director & co-founder of the company, said that its flagship CAR T-cell therapy aimed to transform cancer treatment in India by providing globally advanced, personalised therapies at an affordable cost.
“While this price reflects the complexity of manufacturing personalised CAR T-cell therapy, it is still significantly more affordable compared to similar global options, ensuring access for Indian patients without compromising on quality,” the company told ThePrint.
According to Dr Mukherjee, another board director and co-founder of Immuneel, by combining world-class research CAR-T cell therapy with indigenous manufacturing, the company is “offering new hope to patients facing aggressive blood cancers”.
“By bringing globally benchmarked CAR-T cell therapy to India, we are not only providing a breakthrough treatment but also redefining what is possible in precision medicine. This is a critical step in ensuring that advanced, life-saving therapies are within reach for patients who need them most,” he said.
In October 2023, CDSCO had approved NexCAR19, a CAR-T cell therapy by ImmunoACT, a company incubated at IIT-Bombay and developed in collaboration with Tata Memorial Centre for the treatment of leukemia and refractory or relapsed lymphoma (cancers of the lymph system).
Also read: Cancer warning on liquor bottles ‘long overdue’. Even ‘light’, ‘moderate’ drinking poses threat
Targeting cancer through engineered cells
Immunotherapies for cancer, through which monoclonal antibodies or special types of proteins called antibodies made in laboratories are infused in patients, have been around for more than two decades.
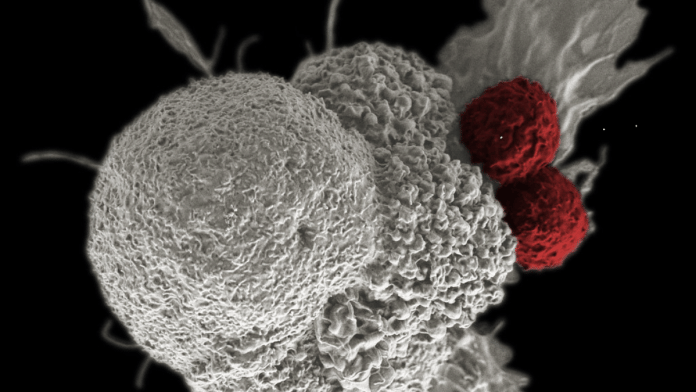
But in the case of CAR T-cell therapy, a type of white blood cells called T-cells are taken out from a patient’s body through a process called apheresis and are genetically engineered to recognise the cancer cells, multiply faster and stay for longer duration in a patient’s body.
Following genetic engineering, during which scientists engineer the T-cells by adding a manufactured CAR (disarmed virus that delivers DNA sequences required to alter T-cells), the converted T-cells (CAR-T cells) can target a specific protein on the cancer cells.
The changed T-cells grow and multiply in the lab and once there are enough cells, patients are given an infusion containing these cells back into their bloodstream. The process can take several weeks and is carried out in a hospital setting during which a patient needs to be constantly under medical supervision.
Both NEXCAR19 and Qartemi – the CAR-T cell therapies approved in the country so far – target CD-19, a specific type of protein biomarker on T-cells.
Qartemi has been approved for the treatment of relapsed or refractory (a non-responsive cancer) B-cell non-Hodgkin lymphoma. The company said the therapy involved modifying a patient’s T-cells to specifically target and destroy cancer cells, offering proven safety and efficacy from global trials.
“Varnimcabtagene autoleucel (CAR-T product used in Qartemi) was licensed from Hospital Clinic de Barcelona, where it was originally developed and has been used for the treatment of patients with B-cell malignancies for over five years,” Immuneel said.
Data from clinical trials in India and Spain show the safety and efficacy of Qartem is similar to that of CAR-T cell therapies approved by the US Food and Drug Administration (USFDA), the company said, adding that the product was manufactured on a closed, automated GMP-manufacturing platform that is the standard for all CAR-T cell therapies approved in the US since 2019.
Immuneel has partnered with over 25 hospitals across the country to provide access to Qartemi to patients in need. The centres include Narayana Health, Apollo Hospitals, Christian Medical College in Vellore & Ludhiana, Manipal Hospitals and Amrita Hospital in Faridabad, among others.
Also read: High fluoride in drinking water lowers IQ in kids, shows data from India, 9 other countries
Rising use of personalised medicine
CAR T-cell therapy for treating specific types of cancer was first approved in the US in 2017 and, as of now, six therapies by various drugmakers have been approved by the USFDA, so far.
As of now, CAR T-cell therapy is approved only for blood cancers in certain cases but research is underway in several countries to assess them for solid cancers. Studies have also been ongoing to develop products that are affordable to patients in developing countries like India.
Clinician-researchers at AIIMS have developed one such CAR-T product targeting B Cell Maturation Antigen (BCMA) protein on T-cell to treat cases of multiple myeloma, a cancer of plasma cells. Clinical trials to test its safety and efficacy are likely to start soon.
Doctors, however, underlined that while CAR T is an effective second or third line treatment for blood cancers, there can also be serious side effects such as cytokine release syndrome (an acute inflammatory response due to a treatment that can be serious or even life-threatening), neurotoxicity, and blood disorders.
In some people, there have also been reports of secondary T-cell malignancies following treatment with CAR T-cell therapies, which prompted the USFDA to include a box warning related to this on all CAR-T cell products beginning January, 2024.
(Edited by Tikli Basu)
Kiran Mazumdar-Shaw is among the founder-investors of ThePrint. Please click here for details on investors.
Also read: Measuring BMI isn’t enough. Lancet panel calls for relook at definition of clinical obesity