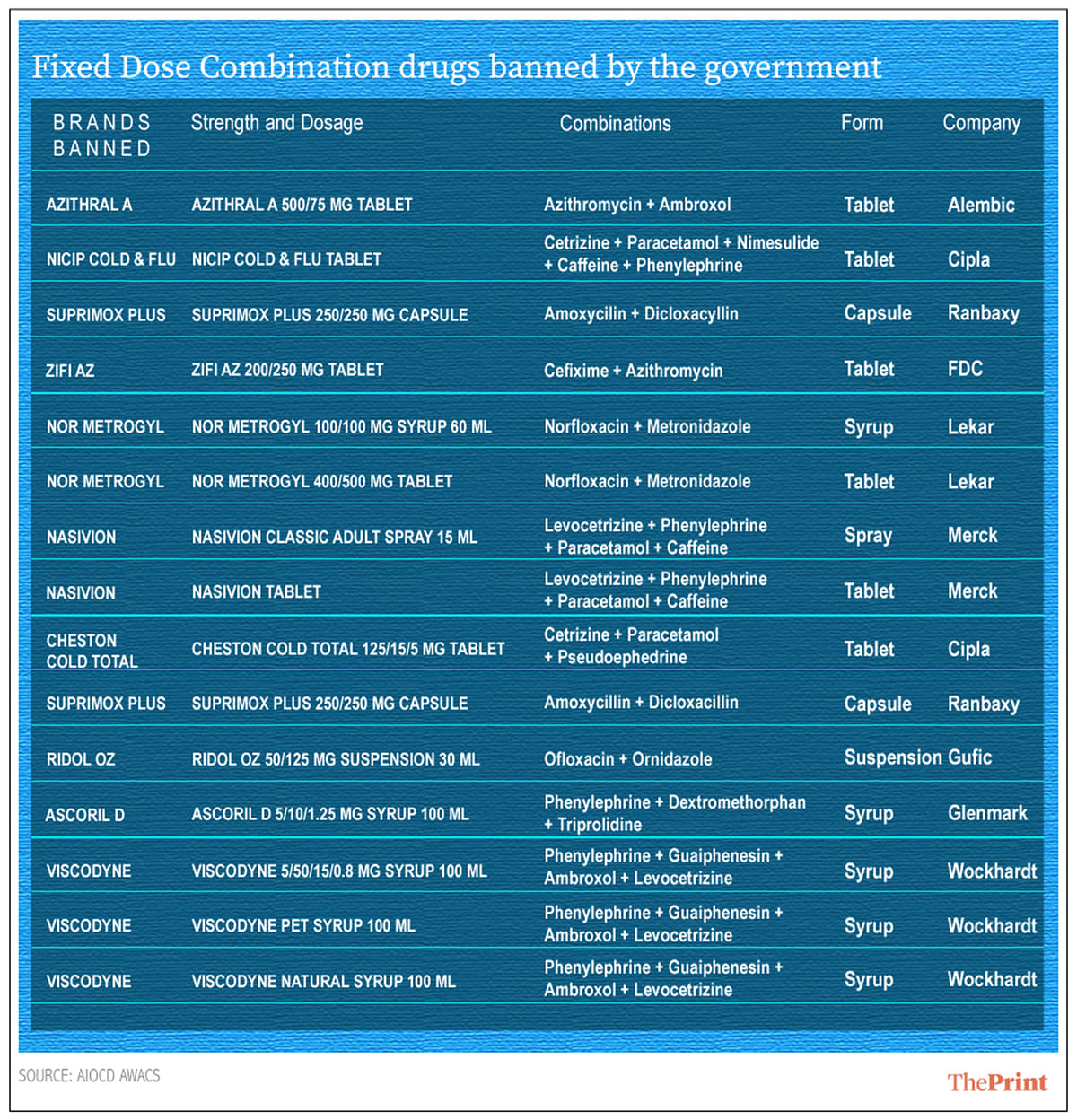
Nasivion Classic Adult Spray, Cheston Cold, Zifi AZ, Nicip among the drugs banned by government.
New Delhi: Chemists have warned of a months-long shortage of antibiotics, analgesics and anti-diabetics, among others, in the wake of a government order banning 328 fixed-dose combinations (FDCs).
Doctors, however, have urged patients not to panic, saying there were enough stocks of alternatives to tide over the bar on these ‘drug cocktails’.
Many common household medicines such as Zifi AZ, Nor Metrogyl, Nasivion Classic Adult spray, Cheston Cold, Total, Azithral A, Nicip Cold & Flu, Suprimox Plus, Ridol Oz, Ascoril D and Viscodyne were among the over 300 FDCs banned last week.
Also read: Banning 328 ‘cocktail’ drugs not a witch hunt, says officer behind the crackdown
A fixed-dose combination contains two or more drugs combined in a fixed ratio of doses, available as a single dose. The government order stems from a bid to end the manufacture of FDCs as they have been found to offer no additional therapeutic advantage, and there is a fear that, in some forms, they end up bolstering resistance.
The health ministry directive, imposed with immediate effect, followed the recommendations of an expert committee formed to examine the efficacy of these drug combinations. The particular stock-keeping units (SKUs) affected by the ban are mentioned in the accompanying table.
Chemists warn of shortages
Following the order, chemists have warned of a shortage of medicines, including generics, in the market.
The All India Organisation of Chemists and Druggists (AIOCD), which represents over 8 lakh medicine sellers across India, has started sending the stocks back to wholesalers, who, in turn, will return the products to the pharma companies.
“We are ready to send back the stock of medicines,” AIOCD president J.S. Shinde told ThePrint.
“Because the notification is valid with immediate effect, customers are likely to face a shortage of products for the next two to three months before we replace these products with the available options,” he added.
“Chemists may also need to shut their shops to clean them and send these medicines back,” he said, “It will take at least 60 days to complete all formalities and return the stocks.”
However, doctors said they were already not prescribing the banned FDCs.
“I have never recommended three-in-one drug combinations, even if they are rational,” said Dr Anoop Misra, an endocrinologist and Fortis C-Doc chairman.
“However, we have used two-in-one rational combinations for the convenience of patients,” he added.
“There is no need for patients to worry as we have a variety of single-dose drugs available as alternatives to the banned FDCs,” he said.
Also read: China’s pollution is a headache for India – could cause a shortage of meds like Crocin
When and why the FDCs are banned?
Spying a big market for FDCs, which were promoted as cheaper and more convenient, drug-makers started flooding the market with several unapproved and non-scientific cocktails, aided by lax regulatory structures.
Their therapeutic value has long been questioned, but experts also fear that the combination of multiple drugs from the same therapeutic group may lead to resistance.
In 2016, the government banned 349 FDC drugs on the ground that they involved a “risk” to humans and there were safer alternatives available.
The manufacturers contested the ban in various high courts and the Supreme Court. Last December, the apex court asked for the matter to be examined by the Drugs Technical Advisory Board (DTAB). Putting an end to a long-drawn legal battle, the board concluded that there was no therapeutic justification for the ingredients in 334 FDCs and recommended banning them.
Business hit
According to research firm AIOCD AWACS, the FDCs that have been banned together have a turnover of Rs 1,040 crore. According to its estimates, the ban impacts over 1,360 medicine brands.
Macleods Pharmaceuticals, with an annual turnover of Rs 292 crore from the banned products, will be worst hit. The others who will take a hit include Mankind Pharmaceuticals (Rs 65 crore), Alkem Laboratories (Rs 58 crore), FDC Limited (Rs 58 crore) and Medley Pharmaceuticals (Rs 41 crore).
The top five therapeutic categories to be impacted include antibiotics, anti-diabetics, analgesics, anti-infectives, and gastrointestinal drugs.



