New Delhi: Researchers in the US have created a detailed, atomic-level model of how the SARS-CoV-2 envelope protein interacts with a human protein that is essential for maintaining the lining of the lungs, which could explain how the virus causes extensive lung damage and goes on to infect other organs, especially in vulnerable Covid-19 patients.
The findings, published in the journal Nature Communications Tuesday, could help speed up the search for drugs that can block the most severe effects of the disease.
“By obtaining atomic-level details of the protein interactions, we can explain why the damage occurs, and search for inhibitors that can specifically block these interactions,” said Qun Liu, a structural biologist at US Department of Energy’s (DOE) Brookhaven National Laboratory.
“If we can find inhibitors, then the virus won’t cause nearly as much damage. That may give people with compromised health a much better chance for their immune systems to fight the virus successfully,” Liu said in a statement.
Also read: Fauci dismissed IIT-Delhi Covid paper as ‘outlandish’ — what it said & why it was withdrawn
Mapping the virus protein & understanding it
Researchers used cryo-electron microscopes that are particularly useful for studying membrane proteins and dynamic protein complexes.
“With this technique, we created a 3D map from which we could see how the individual protein components fit together,” Liu said.
The SARS-CoV-2 envelope protein, which is found on the virus’s outer membrane, helps assemble new virus particles inside infected cells.
It plays a crucial role in hijacking human proteins to facilitate virus release and transmission. Scientists hypothesise that it does this by binding to human cell-junction proteins, pulling them away from their usual job of keeping the junctions between lung cells tightly sealed.
“That interaction can be good for the virus, and very bad for humans — especially elderly Covid-19 patients and those with pre-existing medical conditions,” Liu said.
When lung cell junctions are disrupted, immune cells come in to try to fix the damage. During this process, the cells release small proteins called cytokines. This in turn triggers inflammation, causing the ‘cytokine storm’ and subsequent acute respiratory distress syndrome, which proves to be deadly for many Covid patients.
The weakened cell-cell connections might make it easier for the viruses to escape from the lungs and travel through the bloodstream to infect other organs, including the liver, kidneys, and blood vessels.
Also read: Benford’s Law detects data fudging. So we ran it through Indian states’ Covid numbers
The specs
The scientists obtained atomic-level details of the interaction between envelope (E) protein and a human lung-cell junction protein called PALS1 by mixing the two proteins together, freezing the sample rapidly, and then studying the frozen sample with the cryo-electron microscopes.
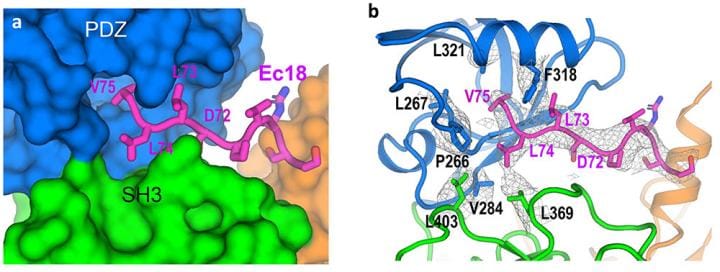
With an overall resolution of 3.65 Angstroms (the size of just a few atoms), the map had enough information about the unique characteristics of the individual amino acids that make up the two proteins. Amino acids are the building blocks of proteins.
“We can see how the chain of amino acids that makes up the PALS1 protein folds to form three structural components, or domains, and how the much smaller chain of amino acids that makes up the E protein fits in a hydrophobic pocket between two of those domains,” Liu said.
The model provides both the structural details and an understanding of the intermolecular forces that allow E proteins deep within an infected cell to wrench PALS1 from its place at the cell’s outer boundary.
(Edited by Manasa Mohan)
Also read: Wuhan lab’s deleted data, unreported pneumonia cases — challenges to ‘natural’ origins of Covid






