New Delhi: Import dependency coupled with quality testing by government labs could postpone the pan-India launch of the Russian-made vaccine Sputnik V by a few more weeks.
While the vaccine first landed in India on 1 May, it was deployed under a ‘limited pilot soft launch’ on 14 May. The objective of this soft launch was to check cold storage arrangements for the Covid-19 vaccine, integration of the vaccine option on the online portal CoWIN and other logistical arrangements before a full-fledged launch.
Under the pilot phase, which was initiated by Dr Reddy’s — the Hyderabad-based pharmaceutical company through which Sputnik V is available in India — the vaccine has been made available in a few hospitals in selected cities.
There are 21 cities where the vaccine is available, including Visakhapatnam, Bengaluru, Mumbai, Kolkata, Delhi, Chennai, Miryalaguda, Vijayawada, Baddi, Kolhapur, Kochi, Raipur, Chandigarh, Pune, Nagpur, Nashik, Coimbatore, Ranchi, Jaipur, Lucknow and Patna.
By the end of the pilot phase, the company aims to reach 28 cities in total.
However, though it’s been seven weeks since the soft launch, a full commercial launch could take some more time.
In an emailed response to ThePrint Wednesday, a spokesperson from Dr Reddy’s admitted that there has been a “slight postponement in the timeline of the commercial launch due to dependency on imported consignments, and quality testing in India”.
However, the company noted, “Our cold storage and other logistical arrangements are being executed in line with our commercialisation plan and are on track with no issues.”
“This pilot phase has allowed us to test our cold storage arrangements of -18 degree C temperature in these cities, CoWIN integration, track-and-trace and other logistical arrangements ahead of our commercial launch,” it said.
“Adequate numbers of cold chain units are being deployed, and the last mile cold chain arrangement is being validated at every partner hospital to ensure seamless storage and handling of the vaccine,” the spokesperson added.
Also read: Cipla gets DCGI nod to import Moderna’s Covid vaccine for restricted emergency use in India
5 crore Sputnik doses to be imported from Russia
Dr Reddy’s has partnered with major hospitals across the country for the distribution of Sputnik V including Apollo, Manipal Hospitals, Fortis Healthcare, Madhukar Rainbow Children’s Hospital and Narayana Health.
Till now, the company has received around 3 lakh doses. It was expecting a third lot of vaccines to arrive from Russia by June, which has not arrived yet.
In total, Dr Reddy’s will be distributing 25 crore doses of Sputnik V in India and the initial 5 crore doses will be imported from Russia before manufacturing can begin in the country for the rest of them.
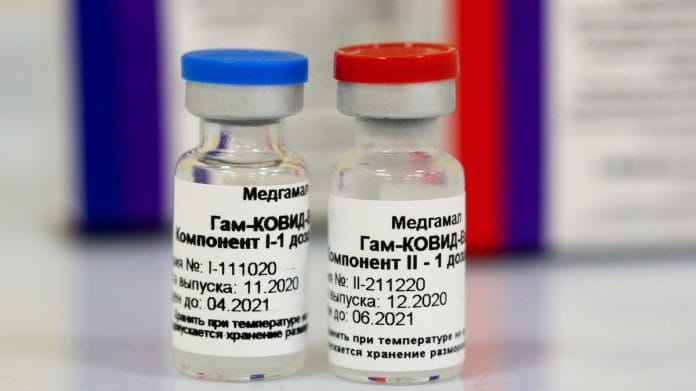
The two-dose Sputnik V is made from two components — recombinant adenovirus 26 (also known as Ad26 or a primer dose) and adenovirus 5 (also known as Ad5, a booster dose).
While Ad26 is the main vaccine, Ad5 is a booster shot which is given 21 days or three weeks apart.
This interval between two doses of a vaccine is shortest for Sputnik. Covaxin’s second dose is administered within a six-week gap and for Covishield, the second shot is administered after 12-16-week gap.
Govt panel denies permission to Sputnik Light trials, says there’s no need
Meanwhile, the Narendra Modi government’s expert panel has rejected a proposal by Dr Reddy’s to begin clinical trials for another version of the Sputnik jab — the one-shot vaccine called Sputnik Light.
The company had applied for testing the vaccine’s immunogenicity, i.e., the ability to trigger an immune response.
While the SEC did not give permission to conduct trials, it asked the company to submit safety, immunogenicity and efficacy data from the Phase 3 clinical trial of Sputnik V in Russia.
The panel also observed that since the safety and immunogenicity data of the first dose of Sputnik V (which is the Sputnik Light) has already been generated by Dr. Reddy’s in India, there was no need for a separate Phase 3 trial.
Also read: There’s a moral & legal case to make vaccines compulsory. Social psychology is another story