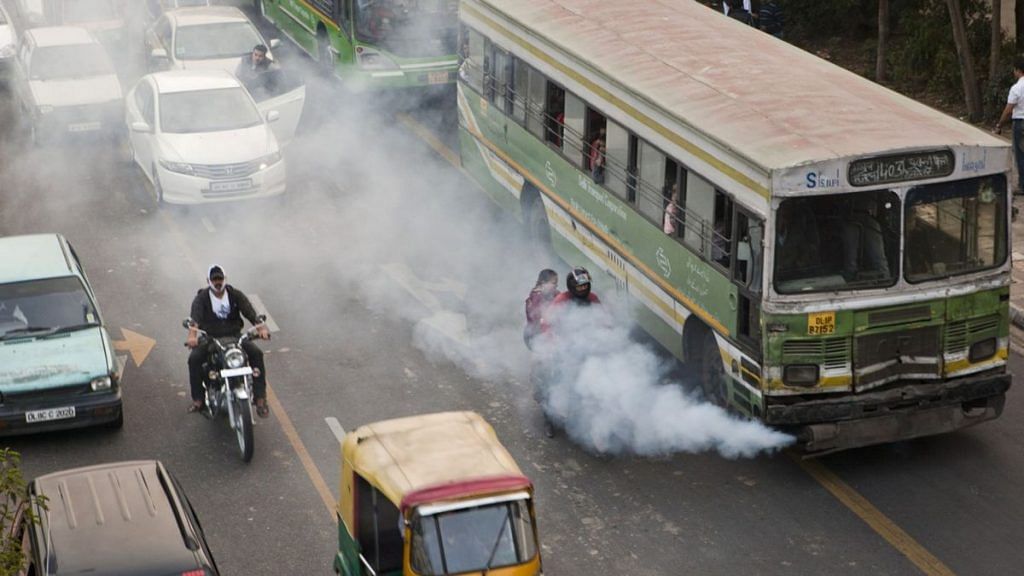New Delhi: A team of scientists at The University of Manchester, UK, has developed a new material that can capture a toxic air pollutant at its source and convert it into a chemical that is used to manufacture fertilisers and rocket propellants.
Named MFM-520, it can selectively capture nitrogen dioxide (NO2), which is an air pollutant, in exhaust gas stream. NO2 is produced when diesel and bio-fuel is burnt.
The material then converts NO2 into nitric acid using only water and air. Nitric acid fuels a multi-billion dollar industry with uses in manufacture of agricultural fertiliser for crops, rocket propellants and nylon.
The metal-organic framework (MOF) material has tiny three-dimensional structures which are porous and can trap gasses inside of it. The internal empty spaces in such materials are vast for their size and just one gram of material can have a surface area equivalent to a football pitch.
“The global market for nitric acid in 2016 was $2.5 billion, so there is a lot of potential for manufacturers of this MOF technology to recoup their costs and profit from the resulting nitric acid production. Especially since the only additives required are water and air,” Sihai Yang, senior lecturer at The University of Manchester, said in a statement.
Also read: Noise pollution threatens survival of over 100 species globally, warns new study
First to capture, convert a toxic pollutant into useful commodity
The material can also capture nitrogen dioxide at ambient pressures and temperatures — even in low concentrations — in the presence of moisture, sulfur dioxide and carbon dioxide. It can be fully regenerated multiple times by degassing or treatment with water — a process that converts nitrogen dioxide into nitric acid.
“This is the first MOF to both capture and convert a toxic, gaseous air pollutant into a useful industrial commodity. It is also interesting that the highest rate of NO2 uptake by this MOF occurs at around 45 degrees Centigrade, which is about the temperature of automobile exhausts,” Yang said.
In the past, capturing greenhouse and toxic gases from the atmosphere was a challenge because of their relatively low concentrations. Water in the air can often negatively affect this process as it becomes difficult to separate water molecules from the targeted gases.
Another problem was finding a practical way to filter out and convert captured gases into useful, value-added products, researchers said. The MFM-520 material offers solutions to many of these challenges.
Also read: Majority of parents in Delhi-NCR want annual ‘smog break’ in schools: Survey