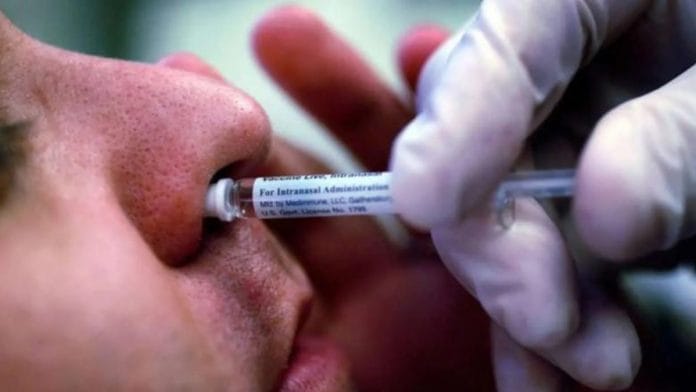
New Delhi: In a first for the US, its Food and Drug Administration (FDA) last week approved a nasal vaccine against influenza—in use for over two decades—for self-administration.
The vaccine FluMist by British-Swedish pharma giant AstraZeneca has been approved for the prevention of influenza disease caused by influenza virus subtypes A and B in individuals aged 2 to 49.
“It is the first vaccine to prevent influenza, more commonly known as the flu, that does not need to be administered by a health care provider,” said the FDA in a statement.
FluMist, the only nasal flu vaccine in the US, was first approved in 2003 for individuals aged 5 to 49. In 2007, its eligibility was expanded to include children as young as 2, with both approvals requiring administration by a vaccine provider.
The vaccine contains a weakened form of live influenza virus strains and is sprayed in the nose, and a prescription is still required to receive FluMist.
However, there are now two approved ways to receive it: Either by a healthcare provider in a medical setting, such as a pharmacy, or by the recipient or a caregiver aged 18 or older.
“The latest approval is a boon as it increases the ease of administration and is likely to be more acceptable to patients. Patients may be more open to taking the vaccine in the comfort of their own homes,” explained Dr Sheetal Chaurasia, consultant, pulmonary medicine, with Manipal Hospital in Bengaluru.
For those interested in self- or caregiver-administration, the vaccine manufacturer plans to make the vaccine available through a third-party online pharmacy.
The development comes even as India’s nasal flu vaccine Nasovac S4—developed by Pune based Serum Institute of India (SII) and marketed and distributed by diagnostic company Mylab—launched last year is yet to find a foothold in the country.
Also read: This experimental lung cancer drug outperforms blockbuster medicine Keytruda. But there’s a catch
Massive disease burden
According to the World Health Organization (WHO), the seasonal flu is an acute respiratory infection caused by influenza viruses. It is common in all parts of the world. And while most people recover without treatment, it causes 3-5 million cases of severe illness, and 290,000 to 650,000 respiratory deaths annually.
There are 4 types of influenza viruses—types A, B, C and D. Influenza A and B viruses circulate and cause seasonal epidemics of disease.
India experiences two peaks of seasonal influenza each year, one between January and March, and another during the post-monsoon season. Dr Sourabh Pahuja, senior consultant, pulmonary medicine, at Amrita Hospital, Faridabad, notes that annual vaccines can reduce the risk of flu by 50-60 percent.
The United Nations health body recommends annual flu vaccinations for pregnant women, children aged 6 months to 5 years, people over 65, those with chronic medical conditions, and healthcare workers.
In India, though, the flu vaccine is not included in the government’s Universal Immunization Programme. With each dose costing between Rs 2,000 and Rs 2,500, this high price is a major factor deterring many from getting vaccinated.
According to the Indian Academy of Pediatrics (IAP)—the largest network of paediatricians in the country—flu vaccines should be taken annually by kids aged between six months and 5 years and those with high-risk conditions.
But in India, the uptake of flu vaccine among children as well as those above the age of 45 has traditionally been low. For example, the Longitudinal Ageing Study in India, a national household survey conducted during 2017–2018, showed that only 1.5 percent of adults aged 45 or above were vaccinated against influenza.
Status of India’s first nasal flu vaccine
Launched in India in November last year, Nasovac S4 is a live quadrivalent influenza vaccine containing four influenza vaccine virus strains, aligning with WHO recommendations, but a dose of 0.5 ml is administered via a syringe and a spray device supplied with the vaccine.
Priced at Rs 1,590 per dose, Nasovac S4 includes two influenza Type A virus strains (A/H1N1 and A/H3N2) and two influenza Type B virus strains (Victoria and Yamagata lineage).
Recommended for individuals above the age of 2, Nasovac S4 actively immunises against influenza disease caused by these virus strains. This nasal spray flu vaccine works by initiating its action in the nasal passages, stimulating the immune system to produce antibodies against the infection.
But Dr Pahuja pointed out that, in India, patients are not yet opting for the nasal vaccine because its indications are very selective. “The availability of this vaccine is also a major concern, as it is a live-attenuated vaccine (which uses a weakened form of the virus to stimulate an immune response),” he said.
The pulmonologist also added that those who take influenza shots still opt for the inactivated vaccine (that contains the killed virus), which is more widely available.
“We can say that the acceptability of the nasal vaccine is not high at present, but with more data emerging in the future, as well as the convenience of its use, it is likely that more people will accept it in due course,” he said.
Dr Vikas Mittal, pulmonologist with CK Birla Hospital in Delhi, too, underlined that nasal influenza vaccine is a recent launch and is yet to become widely available in pharmacies or introduced to doctors.
ThPrint reached SII via email for sale details of Nasovac S4. This report will be updated if and when a response is received.
A spokesperson for Myab, however, replied saying that “The response (to the nasal vaccine) has been great. Acceptance in the paediatric and geriatric segment has been great. Ease and convenience of a needle free vaccine is gaining interest among doctors and patients.”
The company, however, did not share how many doses of the vaccine have been administered in the country so far.
(Edited by Zinnia Ray Chaudhuri)
Also read: Why Indian patients groups are opposing patent requests for blockbuster HIV drug lenacapavir