New Delhi: A study by the Indian Council of Medical Research (ICMR) — the country’s apex medical body — has found that convalescent plasma therapy (CP) as a treatment for Covid-19 has no effect on reducing the disease’s mortality.
“CP was not associated with reduction in mortality or progression to severe COVID-19,” said the study, published in Medrxiv, a pre-print server for health sciences, Tuesday. The study has not been peer-reviewed yet.
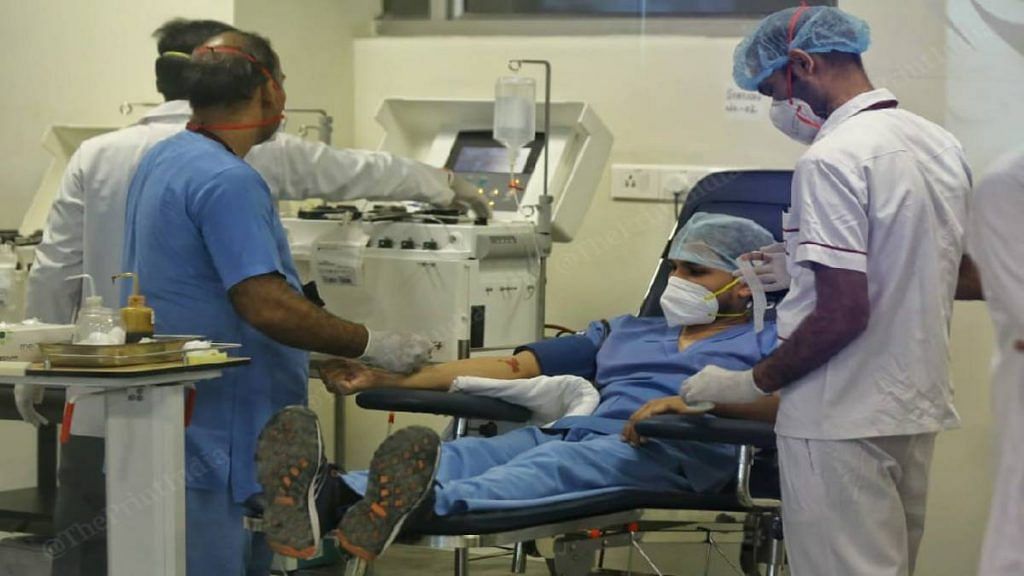
Convalescent plasma therapy for Covid-19 involves the transfusion of a recovered patient’s plasma into someone whose body has not been able to mount a strong enough response against the disease. The idea behind plasma therapy is to leverage the antibodies in a recovered patient’s plasma to help others beat the disease.
The ICMR’s plasma therapy study, called PLACID, is the first and largest randomised control trial to be completed in the world. Two previous studies from China and the Netherlands could not be completed.
Also read: The 9 big states and UTs that beat ICMR to allow Covid tests without prescription
The study
The study involved 464 participants from 39 hospitals, both public and private, across 14 states and union territories in India.
The participants were divided into two groups — the intervention arm of 235 participants received two doses of plasma at a 24-hour interval, while the control arm with 229 participants received “best standard of care” (BSC) without plasma.
All participants were hospitalised with moderate disease.
The primary outcome was measured on the basis of progression to severe disease or possible death, 28 days after enrolment.
Mortality among participants in the intervention and control arms was 13.6 per cent and 14.6 per cent, respectively. In both categories, the disease progressed to severe illness in 7.2 per cent patients in the intervention arm and 7.4 per cent patients in the control arm.
“The PLACID trial results indicate that there was no difference in 28-day mortality or progression to severe disease among moderately ill COVID-19 patients treated with CP along with BSC compared to BSC alone,” noted the report.
It also said the level of antibodies present in the plasma produced “no differences in outcomes”.
While plasma didn’t reduce mortality among patients, the report did find that “the proportion of patients with resolution of shortness of breath and fatigue at day 7 were higher in the intervention arm”.
It also added that the “the negative conversion of SARS-CoV-2 viral RNA at day 7 post-enrollment was significantly higher in the CP arm compared to the BSC arm”.
However, this did not eventually lead to an improvement in mortality or progression to severe illness, according to the study.
ThePrint reached ICMR Director General Balram Bhargava to ask if the study results would change the guidelines for clinical management, but there was no response till the time of publishing. This report will be updated if a response is received.
Also read: Oxford Covid vaccine trial paused after unexplained illness, AstraZeneca says it’s ‘routine’
A contentious treatment
Plasma therapy has been approved for “off-label” use by the Drugs Controller General of India (DGCI), which means the hospitals may charge patients for plasma therapy.
“This authorization has been paralleled by questionable practices such as calls for donors on social media, and the sale of CP on the black market with exorbitant price tags in India,” the study noted, adding that the collection and infusion of plasma is a resource-intensive process “with limited number of institutes in the country having the capacity to undertake these activities in a quality-assured manner”.
ThePrint had earlier reported that the use of plasma therapy, which has for long been considered a life-saving treatment, was going unmonitored.
The ICMR’s study comes weeks after the Food and Drugs Administration (FDA) in the US, the primary drug control agency in the country, granted emergency authorisation to the use of convalescent plasma therapy for Covid-19, allegedly after pressure from President Donald Trump’s administration.
“A priori measurement of neutralizing antibody titres (levels) in donors and participants may further clarify the role of CP in management of COVID-19,” the study said.
Also read: Govt working to help Russia run trials, manufacture Sputnik V Covid vaccine in India