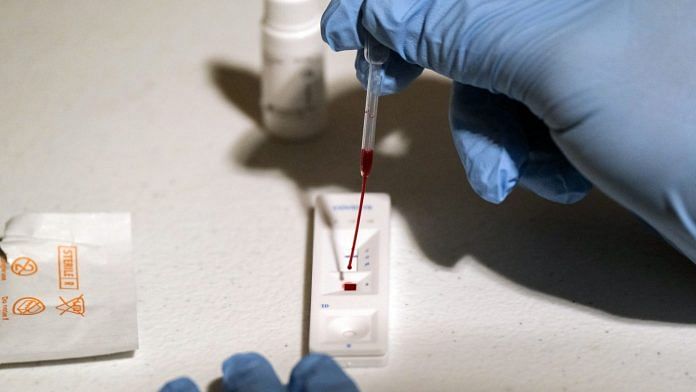
Washington: A 15-minute Covid test from Abbott Laboratories that will be priced at just $5 has been granted emergency authorization for use in the U.S., a breakthrough that could ease the bottleneck that has crimped much of the nation’s testing capacity.
The product, dubbed BinaxNOW, works without relying on laboratory equipment at a time when labs can take as long as two weeks to produce results. It uses a nasal swab and a small reactive card, and it can be administered by a range of health-care workers, including pharmacists, at almost any location.
Abbott will start shipping the test within two weeks and intends to manufacture 50 million tests a month by October. The aim: Meet a surge in demand from Americans seeking to return to in-person schoolrooms and work.
The new test “can be used at a massive scale to help overcome the current waiting game for test results,” said John Hackett, divisional vice president of applied research and technology at Abbott Diagnostics, in a telephone interview.
Abbott shares were up 3.7% to $106.96 in late trading in New York. Through Wednesday’s close, the stock had climbed 19% so far this year.
The test uses so-called lateral flow technology, similar to the method allowing at-home pregnancy tests. Essentially, these tests run a liquid sample along the surface of a pad with reactive molecules to show a result. While a pregnancy test is designed to detect a hormone, Abbott’s BinaxNOW looks for an antigen, a tiny portion of the coronavirus protein that’s collected from inside the nose.
“It’s detecting them at a critical point in the infection cycle, when they are at the highest risk of spreading disease,” Hackett said. “This will be a powerful tool in preventing the transmission of the virus and helping us return to normal life.”
While several other companies are selling antigen tests that also work quickly, they require some equipment to get the results. The technology also sometimes yields lower accuracy in exchange for working faster. The BinaxNOW is 97.1% sensitive, meaning it correctly diagnoses those with the infection that often, and 98.5% specific, meaning it’s correctly ruled out for those without it.
BinaxNOW is the sixth test Abbott has launched to track coronavirus, including molecular tests that detect current infections and antibody tests that show someone has successfully fought off the pathogen. Since the first one was launched in March, the company has sold more than 27 million of its tests so far in the U.S.
At the same time, Abbott is launching a mobile app called Navica that will be connected to the test, giving users an electronic record of their coronavirus status. The results could be used much like a boarding pass to allow those who are negative to return to everyday activities.
Those with a positive result will be told to quarantine and contact their doctor. Health-care workers who conduct the tests are required to report positive results to public health officials.
The U.S. is currently running about 800,000 tests a day nationwide, or 24 million a month, according to the Covid Tracking Project. Abbott built two new manufacturing facilities in the U.S. to produce BinaxNOW, allowing it to more than double the number of tests available to 50 million a month.
“Our nation’s frontline health-care workers and clinical laboratory personnel have been under siege since the onset of this pandemic,” said Charles Chiu, a professor of Laboratory Medicine at University of California, San Francisco. “The availability of rapid testing for Covid-19 will help support overburdened laboratories, accelerate turnaround times and greatly expand access to people who need it.” – Bloomberg
Also read: Oxford Covid vaccine given to 2 volunteers as Serum Institute begins human trials in India